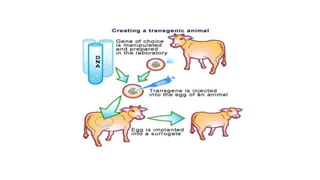
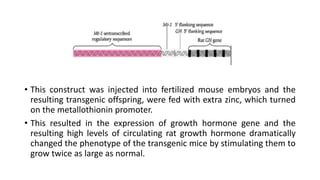
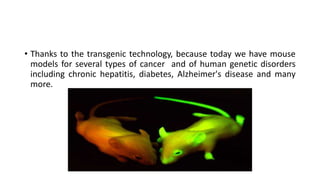
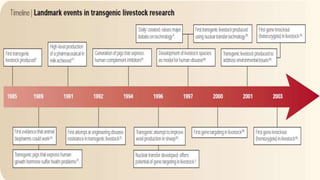
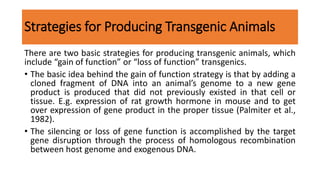
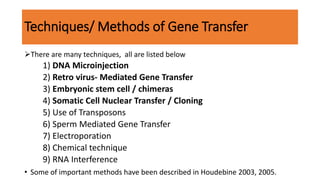
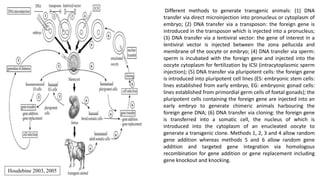
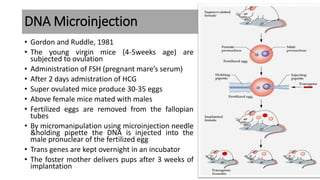
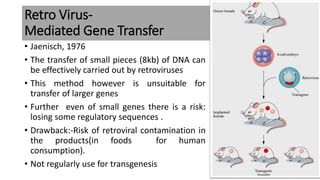
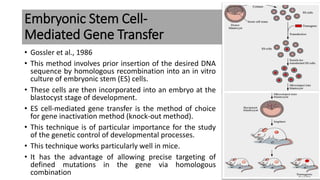
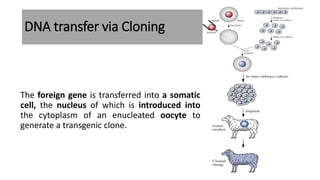
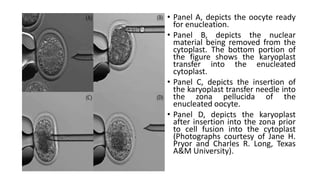
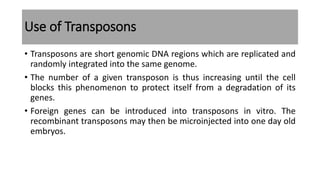
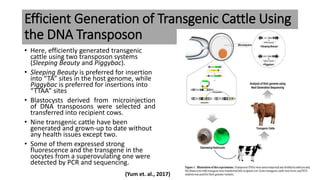
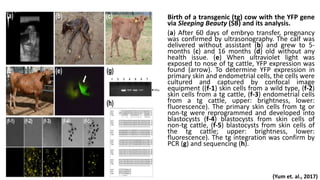
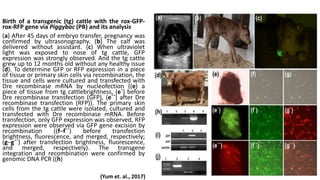
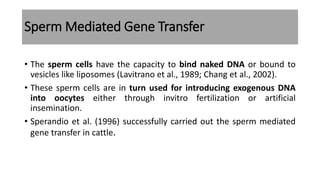
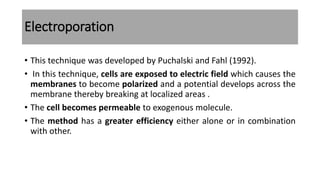
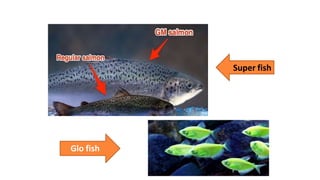
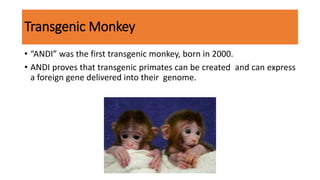
The document discusses reproductive biotechnology with a focus on transgenesis, the process of introducing foreign DNA into an animal's genome to create transgenic animals that exhibit new properties and transmit them to their offspring. It outlines historical developments, various gene transfer techniques, and examples of transgenic animals, emphasizing their importance in medical research, agriculture, and industry. Ethical concerns and limitations related to transgenic technology are also addressed.