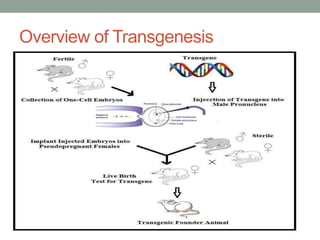
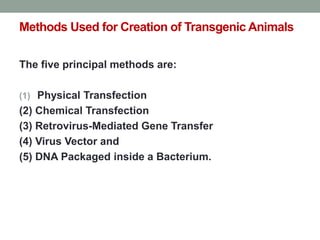
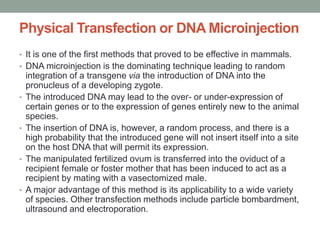
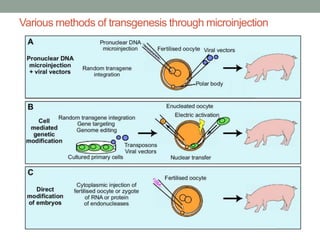
The document discusses transgenesis, the methods for creating transgenic animals, and their applications in biomedical research. It outlines various techniques such as DNA microinjection, chemical transfection, and retrovirus-mediated gene transfer used to manipulate genes for research purposes. Additionally, it highlights the significance of transgenic animals like Dolly the sheep and transgenic mice in studying diseases, developing biological products, and testing vaccine safety.






![DNA Packaged inside a Bacterium
(Bactofection):
• Generally, Agrobacterium tumefaciens mediated transfer of
DNA is a common practice in plant system.
• It has been shown by Kunik and co -workers (2001) that this
bacterium can transfer DNA in cultured human cells.
• It was established in mid-1990 that several bacteria infect
human cells and undergo lysis releasing plasmid in host cells
e.g., Salmonella spp., Listeria spp. and Shigella spp.
• The plasmid DNA then finds its way to the host cell nucleus,
where it is integrated in the genome and expressed.
• Another method of DNA transfer is by conjugation [the transfer
of DNA through a pilus (plural pilli)]. This pilus is formed by
bacterial cell.
• When live bacteria are used, it is necessary that the bacteria
are attenuated. This is because the gene transfer system uses
the natural ability of bacteria to infect eukaryotic cells. The
bacteria may multiply and destroy host cells.](https://image.slidesharecdn.com/transgenesismethodsandapplications-200408133918/85/Transgenesis-methods-and-applications-14-320.jpg)





