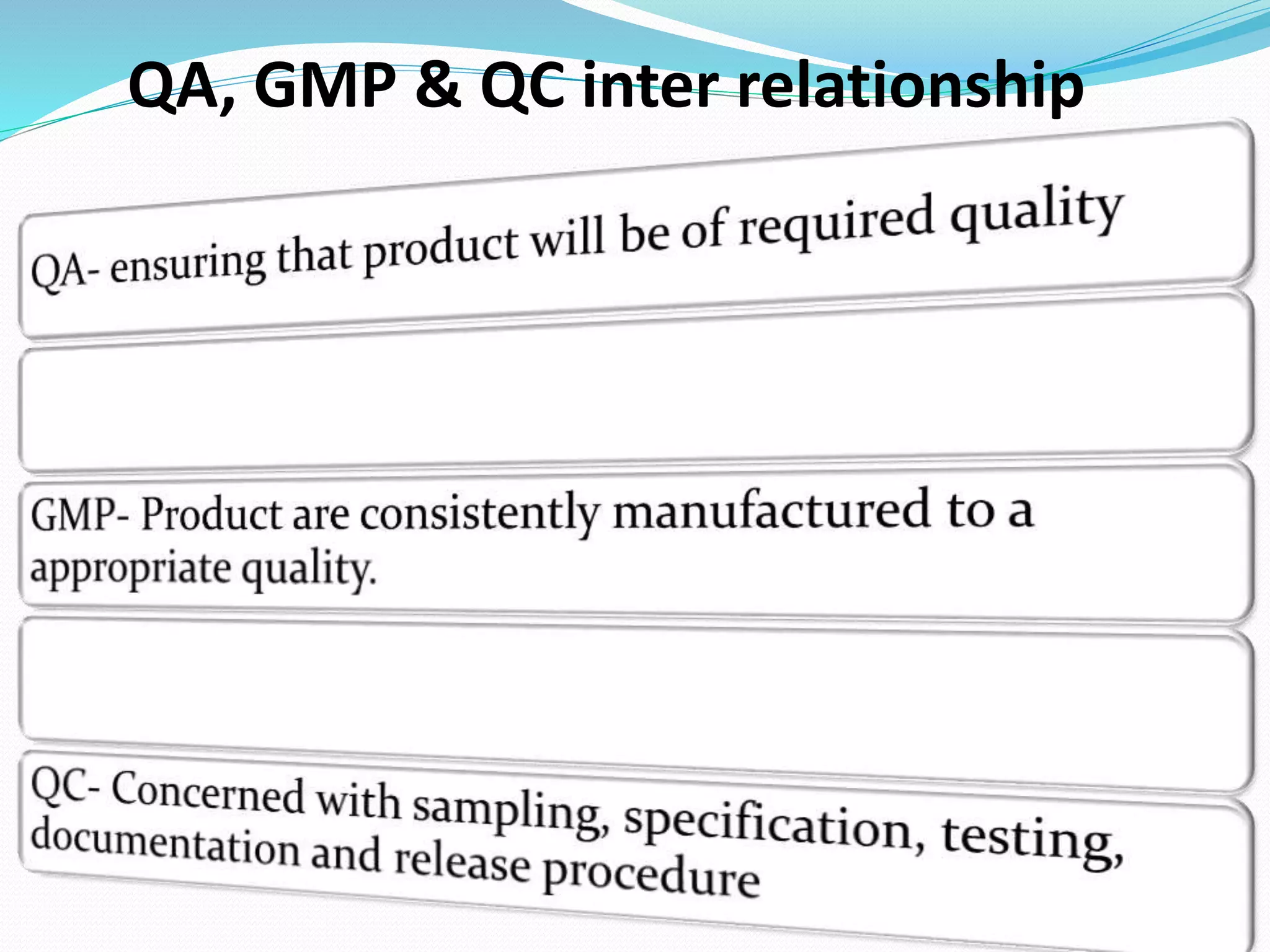
Quality assurance and good manufacturing practices are important to ensure drug quality and safety. Quality assurance covers all factors that influence a product's quality and requires adequate resources. Good manufacturing practices provide quality standards for production and controls to ensure products meet their intended use as required by regulations. Key elements of good manufacturing practices include facilities, equipment, documentation, materials management, production controls, packaging and labeling, storage, and validation. While GMPs vary between countries, the overall goals are similar in requiring design and maintenance standards, standard operating procedures, quality control, trained personnel, and management oversight.