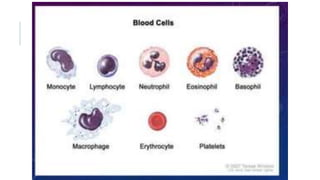
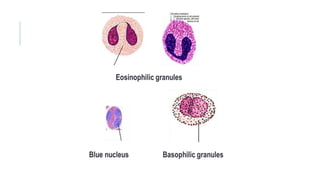
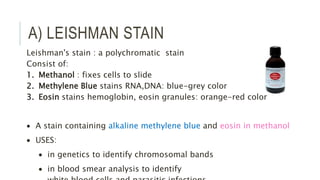
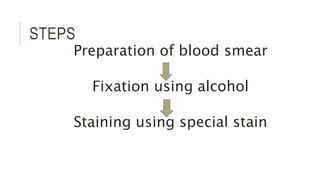
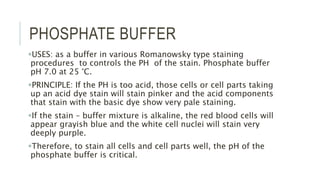
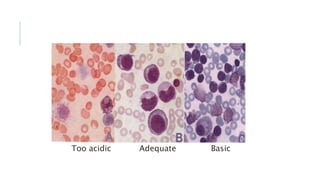
Romanowsky stains are commonly used to stain blood films. They contain basic dyes like methylene blue that stain nucleic acids blue-grey and acidic dyes like eosin that stain proteins and granules orange-red. Leishman's stain contains methylene blue and eosin in methanol and is useful for identifying cells in blood smears and genetics. The staining process involves making a blood smear, fixing it with alcohol, staining it using the Romanowsky stain followed by a rinse to differentiate cells by their staining. Care must be taken to maintain the proper pH and timing during staining.