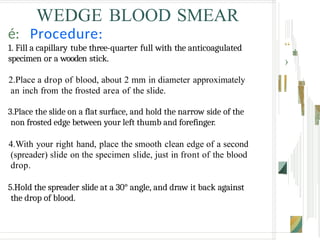
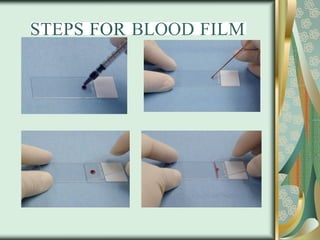
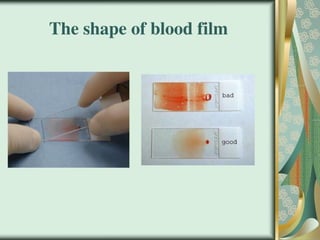
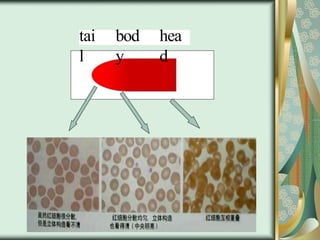
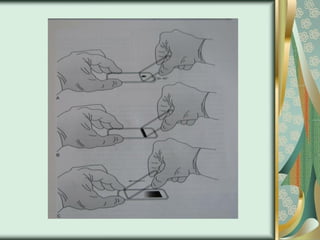
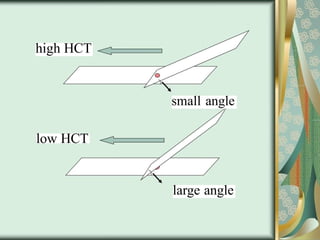
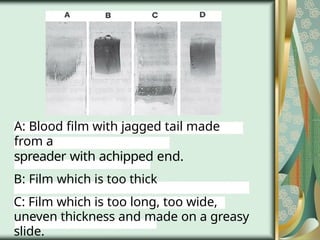
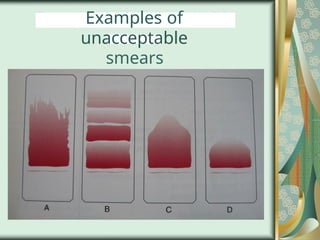
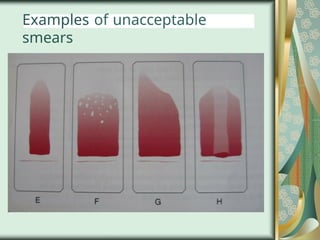
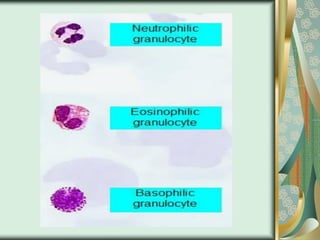
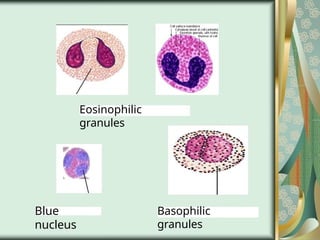
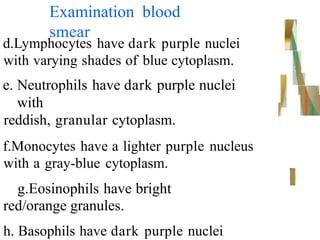
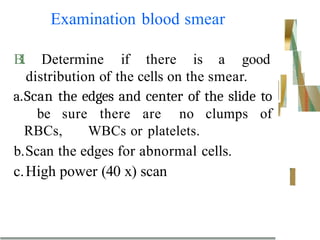
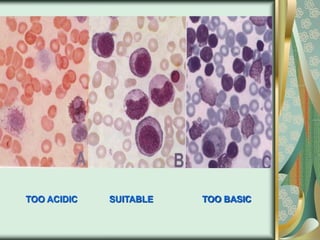
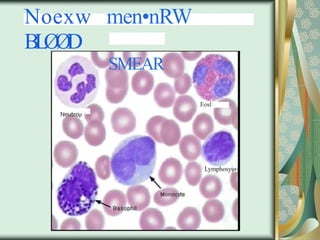
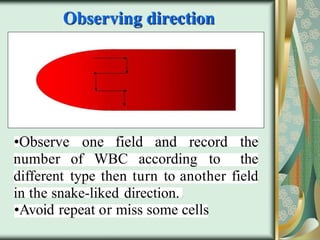
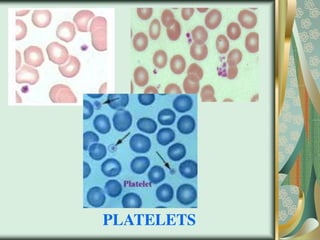
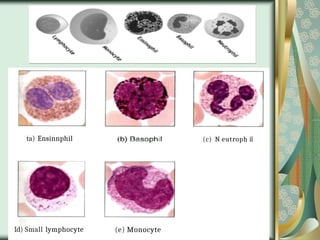
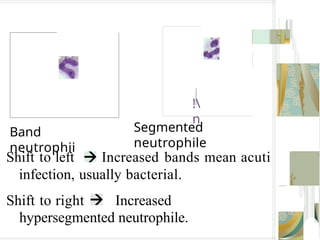
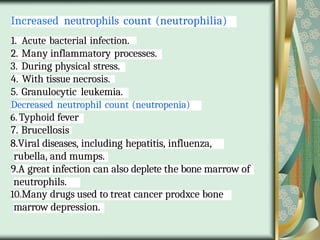
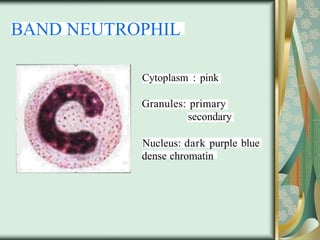
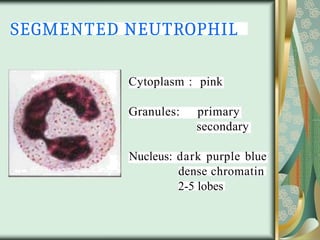
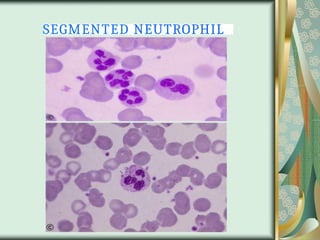
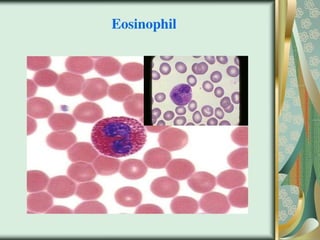
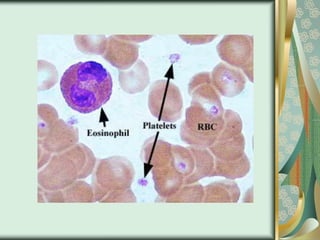
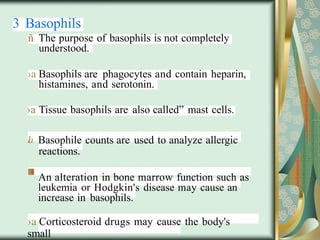
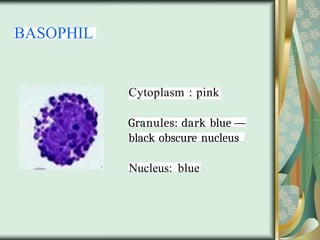
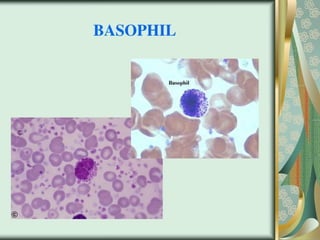
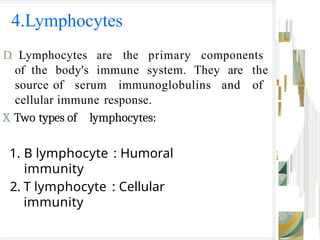
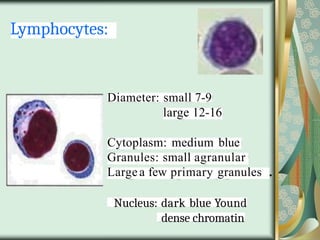
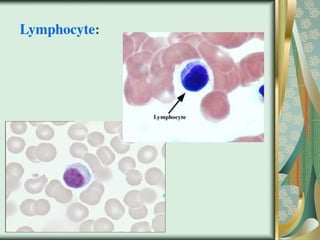
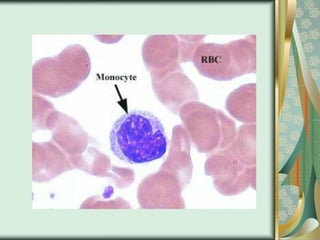
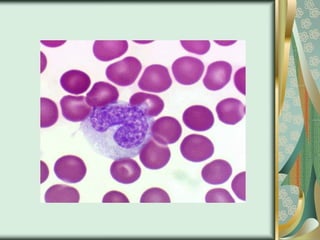
The document outlines the blood smear preparation and staining processes, which are crucial for diagnosing conditions such as anemia and infections. It details the steps involved in creating a blood smear, fixing, and staining, emphasizing factors that affect smear quality and staining results. Additionally, it explains the importance of analyzing white blood cell types and abnormalities through microscopy for diagnostic purposes.