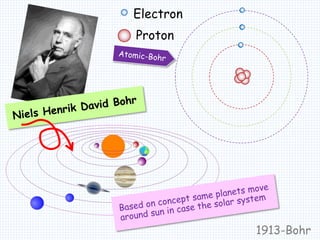
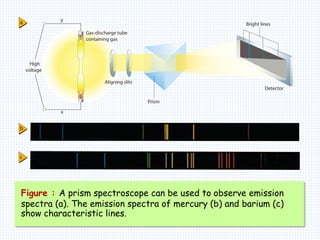
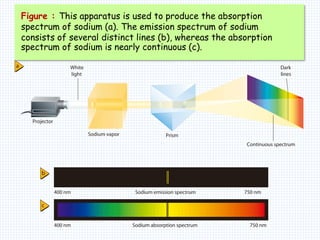
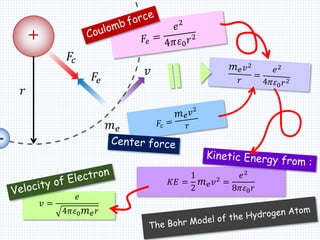
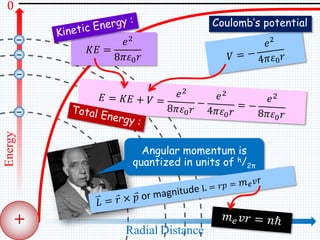
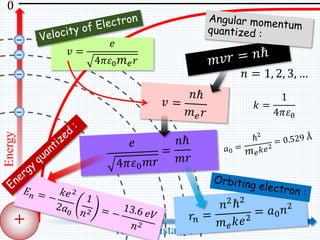
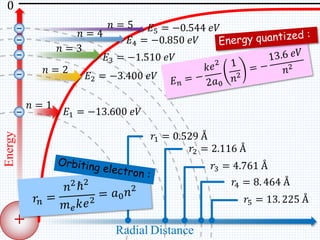
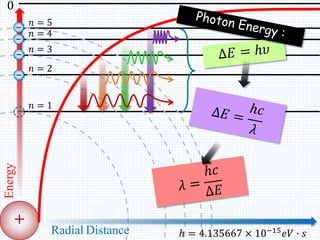
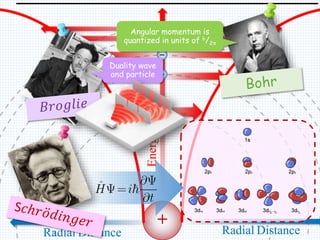
1. In 1913, Niels Bohr proposed an atomic model of the hydrogen atom in which the electron orbits the nucleus in fixed, quantized energy levels.
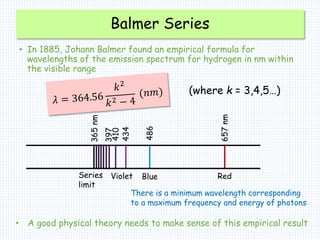
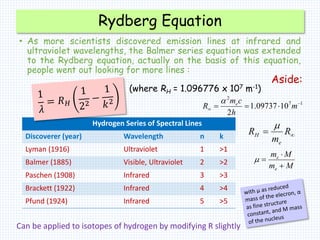
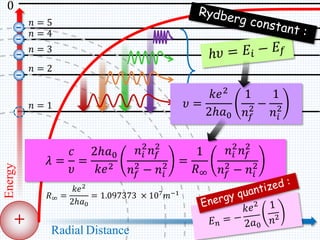
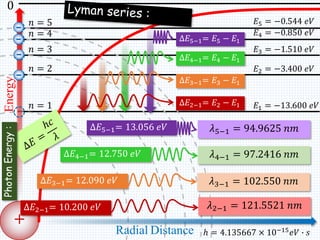
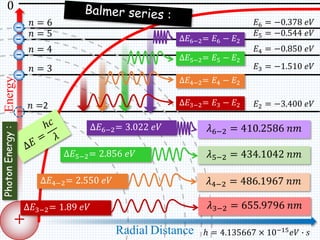
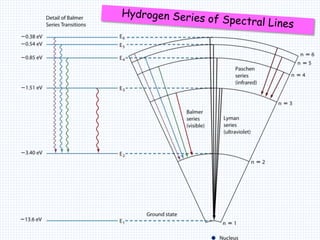
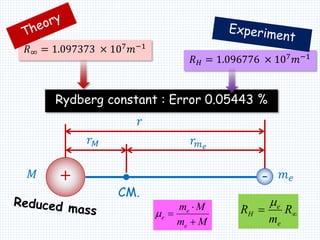
2. Bohr's model explained the empirical formulas discovered by Balmer and Rydberg for the emission spectrum of hydrogen. It predicted that only certain orbital radii and electron energies were allowed.
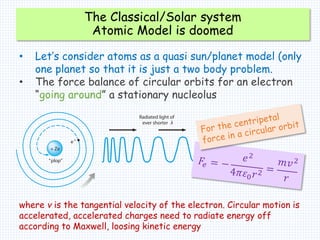
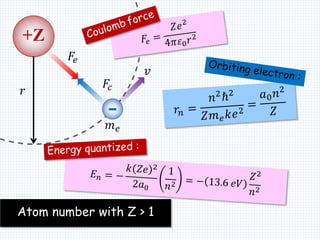
3. Bohr's model resolved issues with the classical model, which predicted electrons would radiate energy and spiral into the nucleus. By quantizing angular momentum, Bohr was able to explain the stability of atoms.
![2A:B
B
Hydrogen
2A
Oxygen
Watter[H2O]](https://image.slidesharecdn.com/timelineofatomicmodels-180125044335/85/Timeline-of-atomic-models-3-320.jpg)
![-
+
-
+
+-
-+ +
-
1897-J.J.Thomson
Helium gas
[-] Negative Charge
[+] Positive Charge
Unlike-sign charges attract.
e/me = - 1.758821011 C/kg
J.J. Thomson's experiment and the
charge-to-mass ratio of the electron](https://image.slidesharecdn.com/timelineofatomicmodels-180125044335/85/Timeline-of-atomic-models-4-320.jpg)
![[+] Proton
[-] Electron
1907-Rutherford](https://image.slidesharecdn.com/timelineofatomicmodels-180125044335/85/Timeline-of-atomic-models-5-320.jpg)
![Robert A. Millikan
-
-
-
Fg = mg
Fe = qE
1910-Millikan oil drop
e/me = - 1.758821011 C/kg
J.J. Thomson's experiment and the
charge-to-mass ratio of the electron
Newton's first law :
SF = 0 [Forces are Balanced]](https://image.slidesharecdn.com/timelineofatomicmodels-180125044335/85/Timeline-of-atomic-models-6-320.jpg)