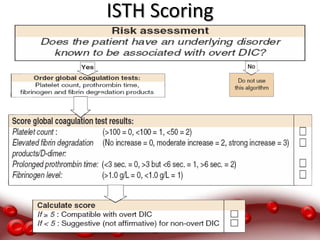
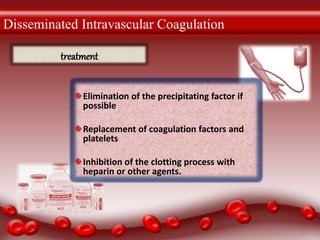
The document discusses various thrombotic disorders including:
1. Congenital and acquired thrombotic disorders are characterized by the formation of blood clots that obstruct blood flow.
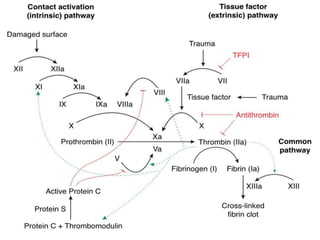
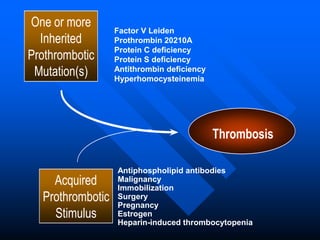
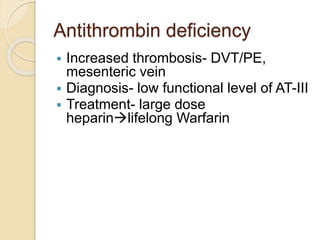
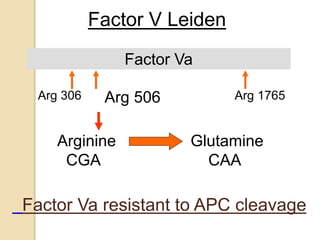
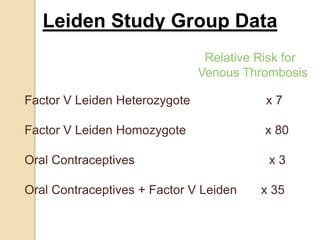
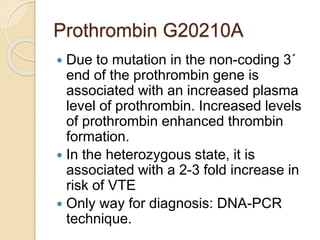
2. Some inherited prothrombotic mutations that can cause thrombosis include Factor V Leiden, prothrombin 20210A, and deficiencies of antithrombin, protein C, and protein S.
3. Acquired conditions like malignancy, immobilization, surgery, pregnancy, estrogen use, and heparin-induced thrombocytopenia can also trigger blood clots when combined with inherited mutations.