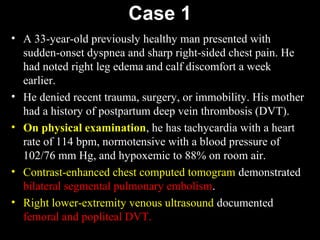
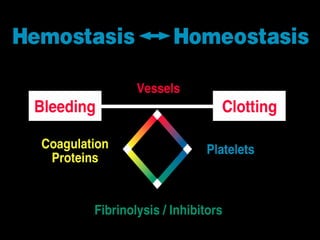
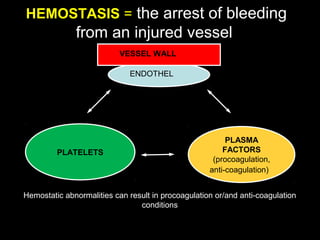
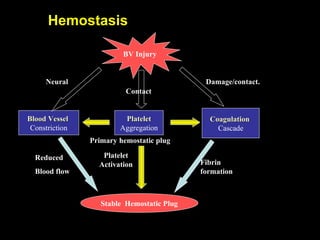
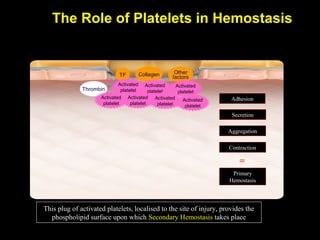
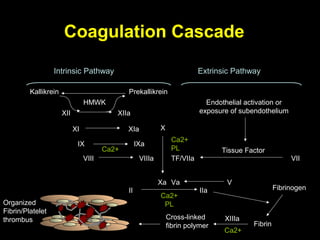
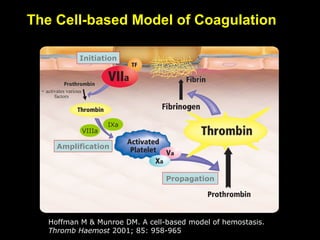
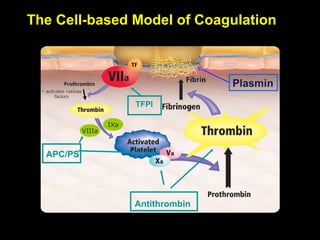
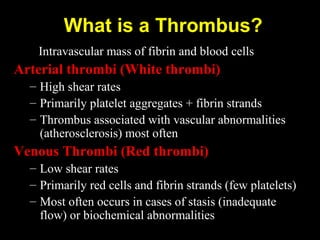
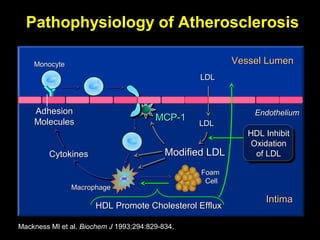
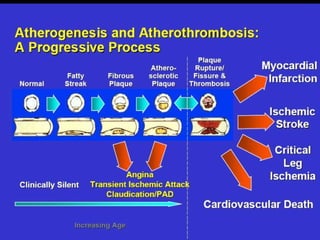
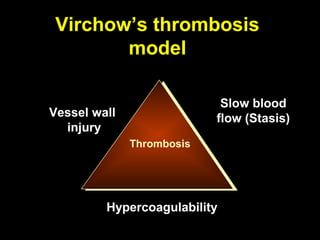
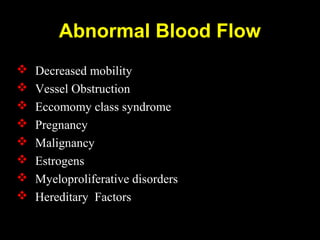
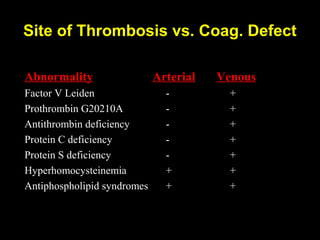
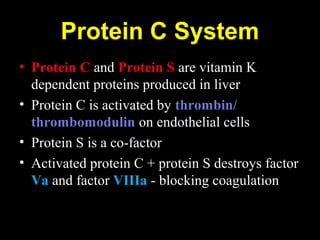
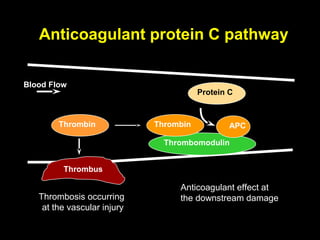
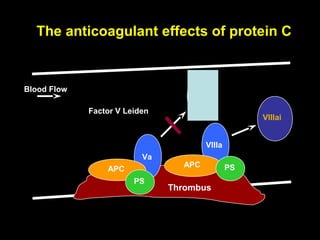
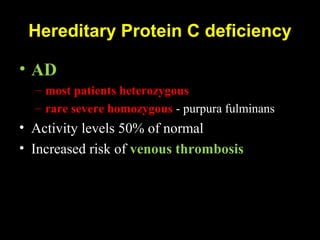
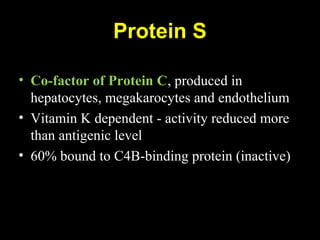
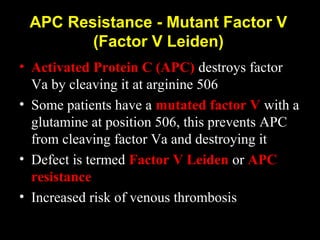
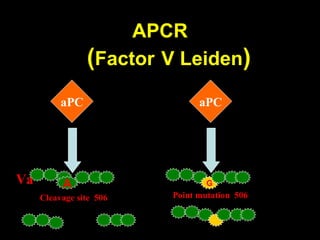
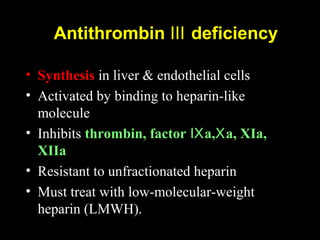
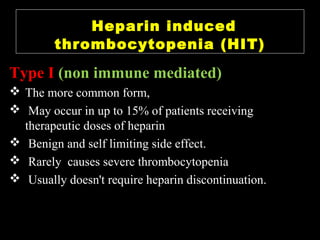
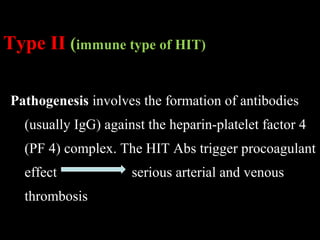
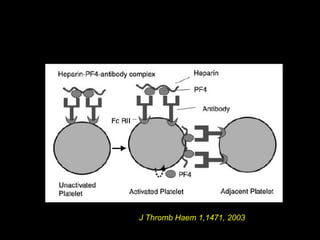
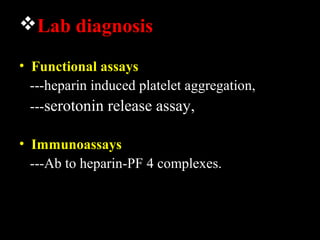
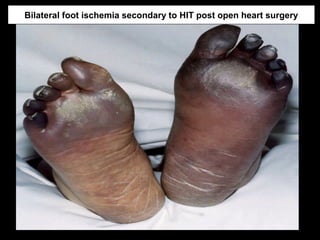
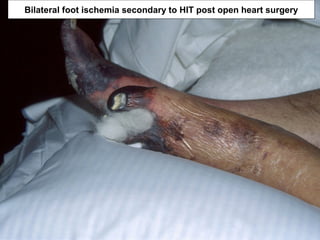
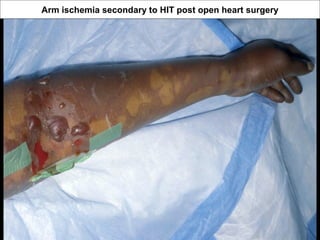
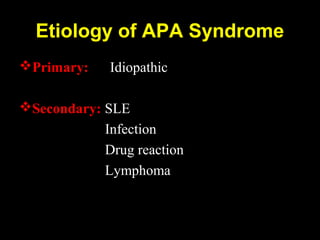
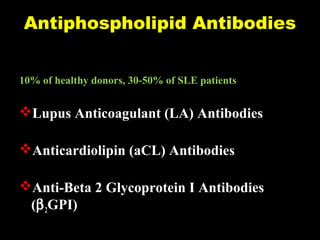
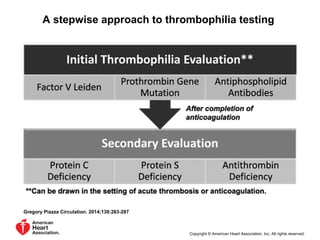
This document discusses hypercoagulable states (thrombophilia). It presents two case studies of patients presenting with deep vein thrombosis (DVT). It then defines thrombophilia as a disorder associated with an increased tendency to form blood clots. The document reviews hemostasis and coagulation mechanisms, inherited and acquired risk factors for hypercoagulability, and recommends a stepwise approach to thrombophilia testing that considers the clinical scenario and implications of testing.