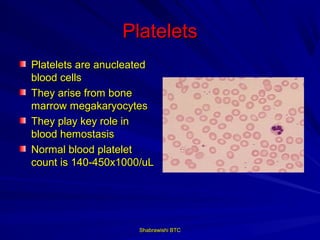
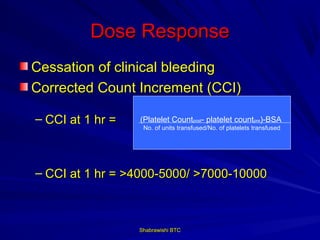
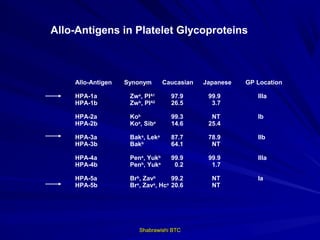
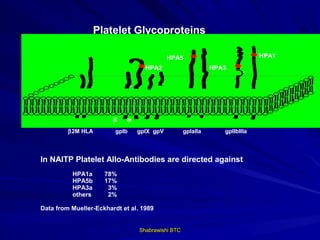
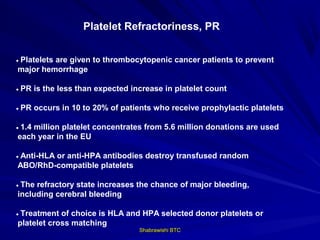
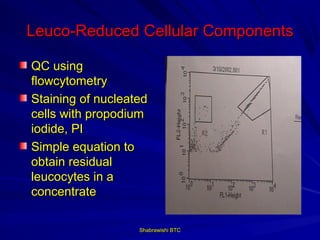
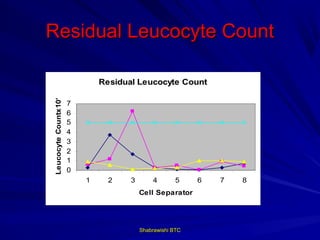
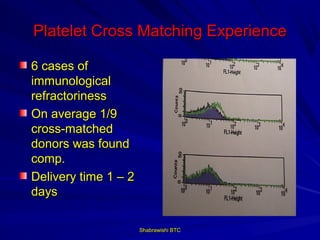
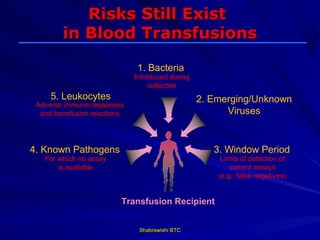
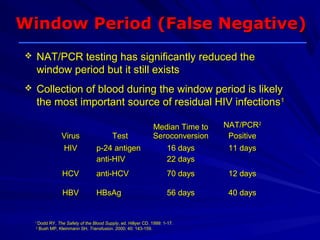
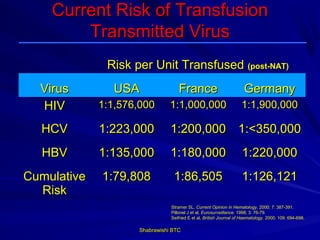
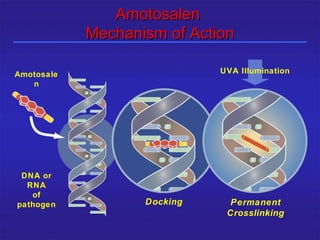
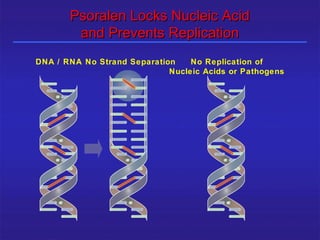
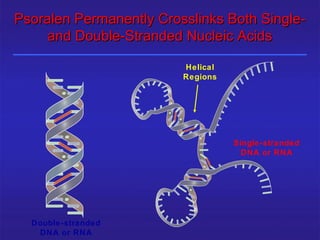
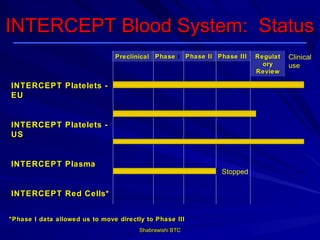
This document provides information about platelet transfusion. It discusses what platelets are, their role in hemostasis, normal platelet counts, causes of thrombocytopenia, indications for platelet transfusion, contraindications, donor criteria, preparation of platelet concentrates, dosing, response to transfusion, complications including immunological and non-immunological issues, and methods to reduce complications like use of leukoreduced products. The document contains detailed information about platelet immunology, causes of refractoriness, its management, and methods to improve safety and availability of platelet transfusion like use of matched donors and crossmatching.