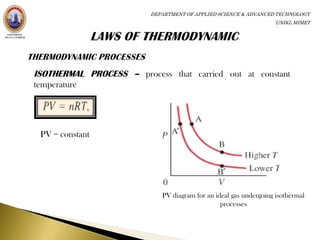
This document discusses thermodynamics and heat engines. It covers the laws of thermodynamics, including the first law that states energy is conserved as it is transferred or transformed, but not created or destroyed. It also discusses the second law which states heat cannot spontaneously flow from a cold to a hot body. The document describes heat engines and their components. It provides examples of heat engines like steam engines, turbines, and internal combustion engines. It also discusses the efficiency of heat engines.









![DEPARTMENT OF APPLIED SCIENCE & ADVANCED TECHNOLOGY
UNIKL MIMET
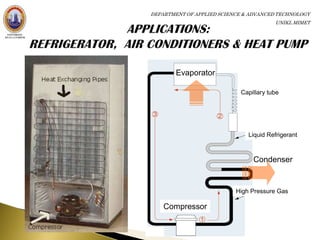
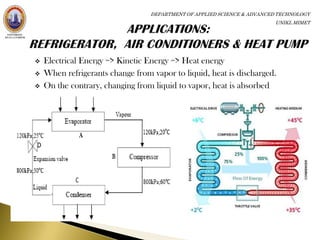
The principle of refrigerators, air conditioners and heat pumps is just the
reverse of a heat engine.
Refrigerator: no work is required to take heat from the low-temperature
region to high-temperature region [no device is possible whose sole effect
is to transfer heat from one system at a temperature TL into a second
system at a higher temperature TH.]
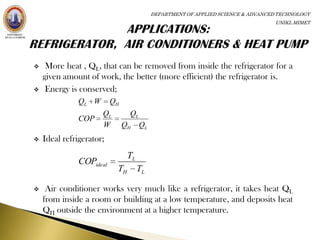
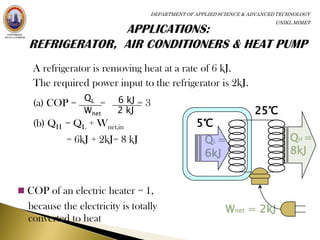
The coefficient of performance (COP) of a refrigerator is defined as the
heat QL removed from the low-temperature area divided by the work
done W to remove the heat.
QL
COP [refrigerator and
W air conditioner]](https://image.slidesharecdn.com/thermodynamics-120422095454-phpapp01/85/Thermodynamics-22-320.jpg)