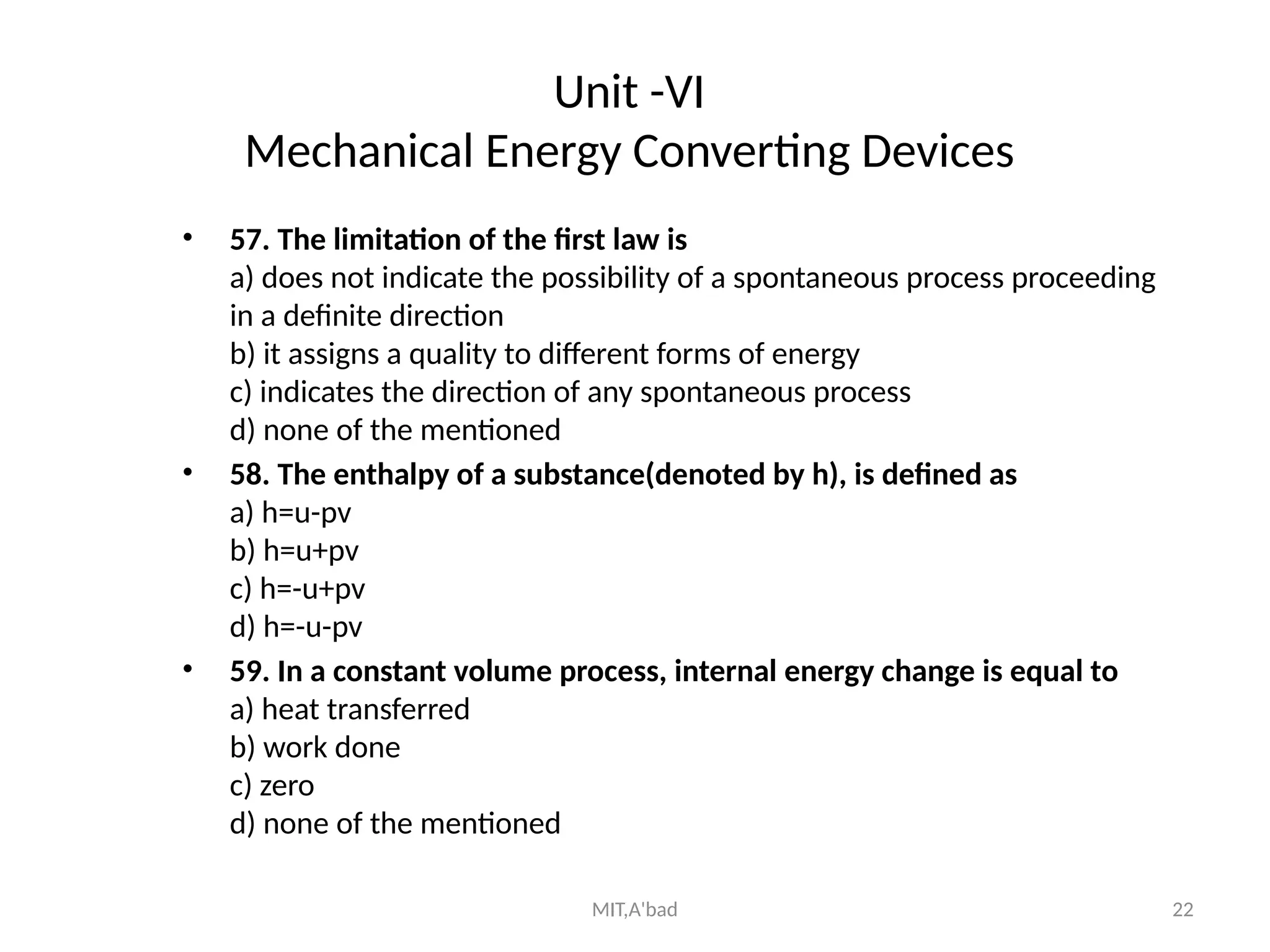
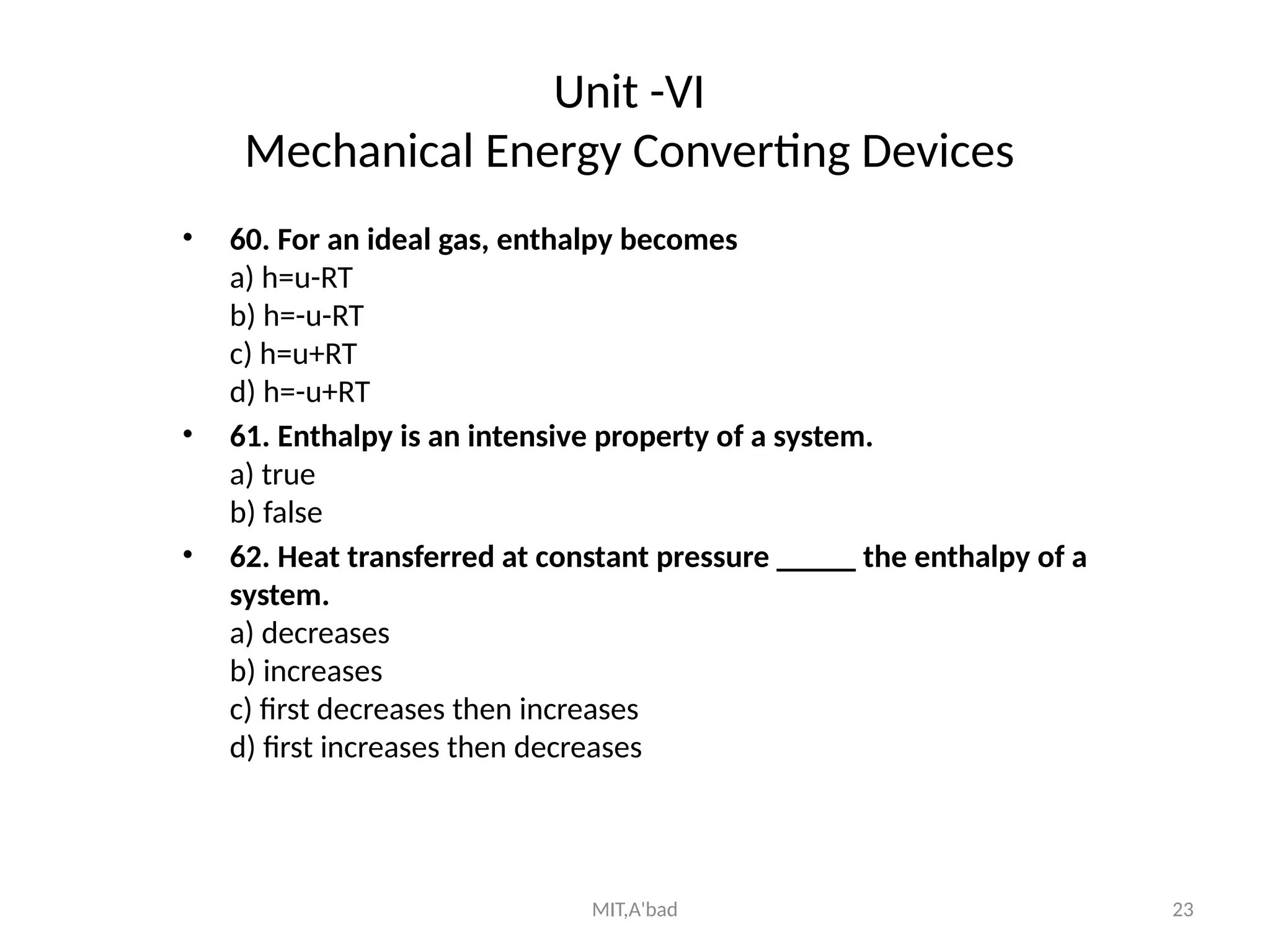
This document outlines the fundamentals of thermodynamics and mechanical engineering, covering various topics including laws of thermodynamics, ideal and real gases, properties of steam, fuels, and combustion. It contains questions and concepts related to thermodynamic principles, mechanical energy devices, heat transfer, and the first law of thermodynamics. The document serves as a comprehensive guide for understanding the basic principles and calculations in mechanical engineering.