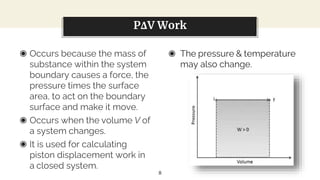
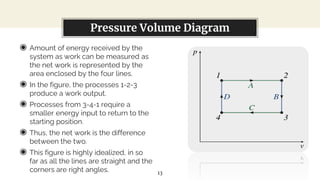
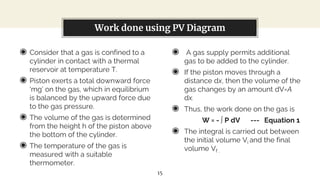
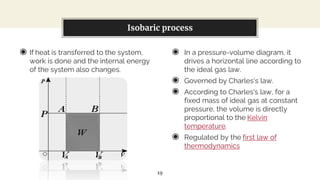
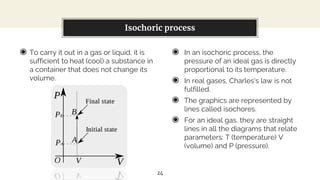
This presentation discusses thermodynamic work and processes involving changes in pressure and volume. It begins by defining work as the energy transferred by a system to its surroundings. Work can be measured in joules or newton-meters. Pressure-volume work occurs when the volume of a system changes and is represented by the area under the pressure-volume curve. An isobaric process maintains constant pressure, while an isochoric process maintains constant volume. Pressure-volume diagrams are used to visualize these processes and calculate work done on a system based on changes in pressure and volume.