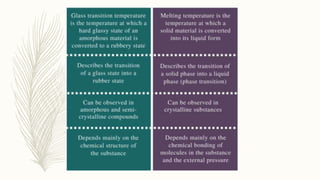
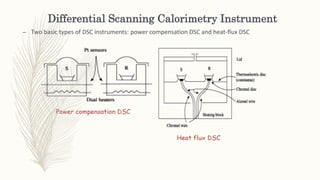
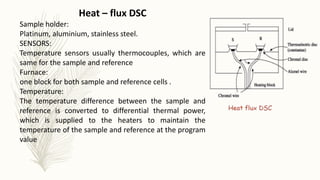
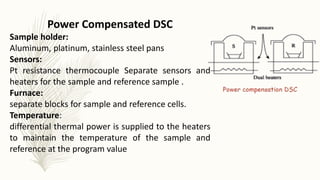
This document discusses thermal analysis techniques such as differential scanning calorimetry (DSC). DSC measures the difference in heat flow between a sample and reference material as they are heated. It is used to study thermal transitions in polymers like melting, glass transition, and crystallization. The document outlines the principles and components of a DSC instrument, sample preparation procedures, factors affecting DSC curves, and applications of DSC in fields like polymers, ceramics, and pharmaceuticals.