This document discusses chemical bonding and Lewis structures. It explains that valence electrons are involved in bonding and give atoms their chemical properties. Electron-dot diagrams called Lewis structures represent the valence electrons around an element symbol. The octet rule states that atoms want 8 electrons in their outer shell, which they achieve by gaining, losing or sharing electrons to acquire a noble gas configuration. There are two main types of bonds: ionic bonds form when valence electrons are transferred between atoms, and covalent bonds form when valence electrons are shared between atoms.








![Lewis Structures
Ne:
:..
..
1s22s22p6 = [Ne]
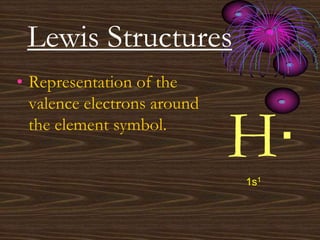
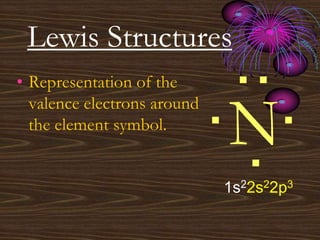
• Representation of the
valence electrons around
the element symbol.](https://image.slidesharecdn.com/unit9powerpoint-100208154700-phpapp02-230426014015-1cf001fa/85/unit9powerpoint-100208154700-phpapp02-pdf-12-320.jpg)
![Lewis Structures
Ar:
:..
..
1s22s22p63s23p6
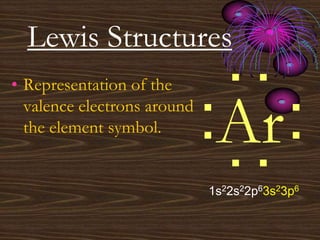
• Representation of the
valence electrons around
the element symbol.
1s22s22p6 = [Ne]](https://image.slidesharecdn.com/unit9powerpoint-100208154700-phpapp02-230426014015-1cf001fa/85/unit9powerpoint-100208154700-phpapp02-pdf-14-320.jpg)
![Lewis Structures
Ar:
:..
..
1s22s22p63s23p6
[Ne]3s23p6
• Representation of the
valence electrons around
the element symbol.
1s22s22p6 = [Ne]](https://image.slidesharecdn.com/unit9powerpoint-100208154700-phpapp02-230426014015-1cf001fa/85/unit9powerpoint-100208154700-phpapp02-pdf-15-320.jpg)
![Lewis Structures
Ca·
·
1s22s22p63s23p64s2
• Representation of the
valence electrons around
the element symbol.
[Ar] = 1s22s22p63s23p6](https://image.slidesharecdn.com/unit9powerpoint-100208154700-phpapp02-230426014015-1cf001fa/85/unit9powerpoint-100208154700-phpapp02-pdf-16-320.jpg)
![Lewis Structures
Ca·
·
1s22s22p63s23p64s2
[Ar]4s2
• Representation of the
valence electrons around
the element symbol.](https://image.slidesharecdn.com/unit9powerpoint-100208154700-phpapp02-230426014015-1cf001fa/85/unit9powerpoint-100208154700-phpapp02-pdf-17-320.jpg)








