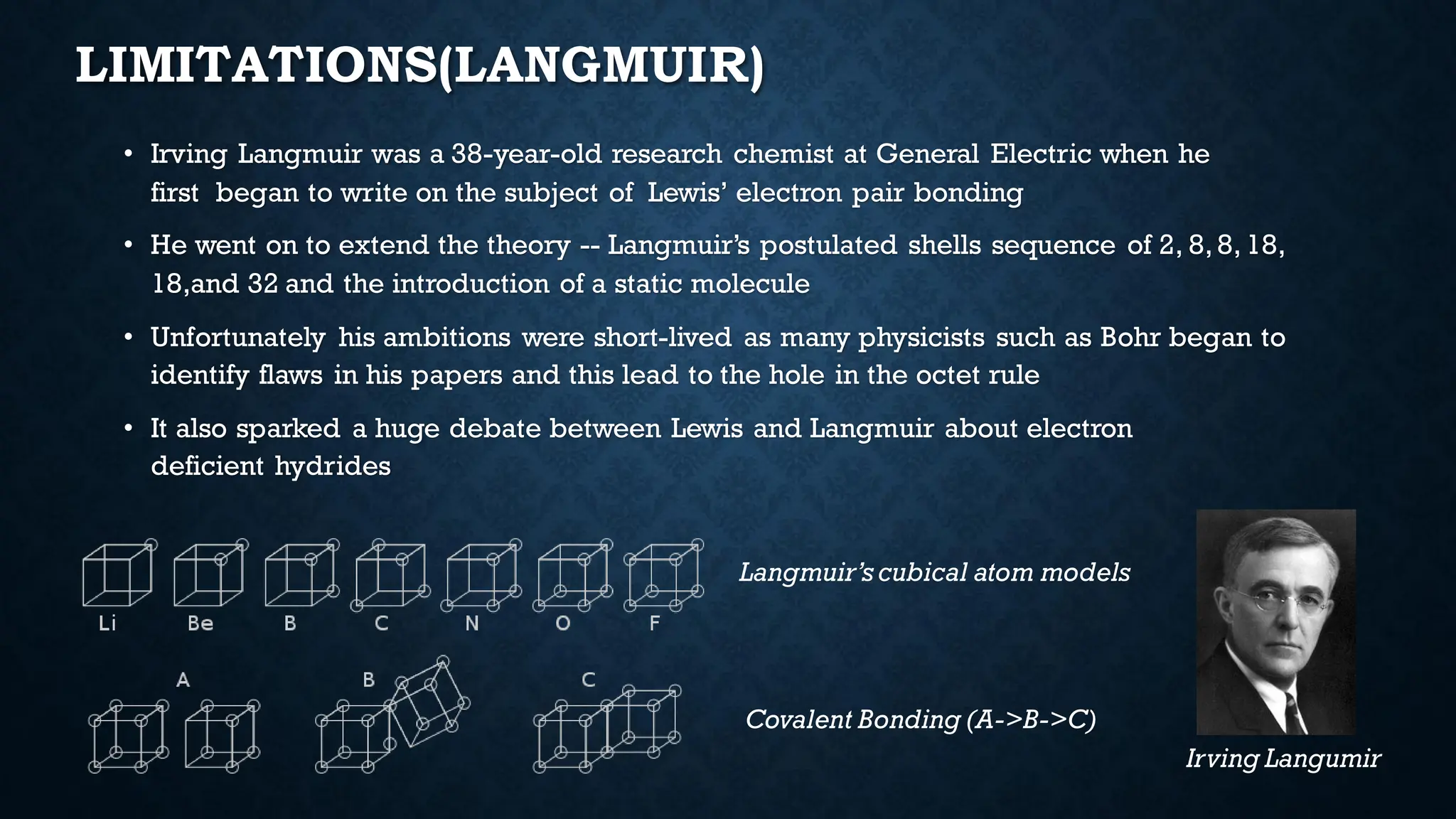
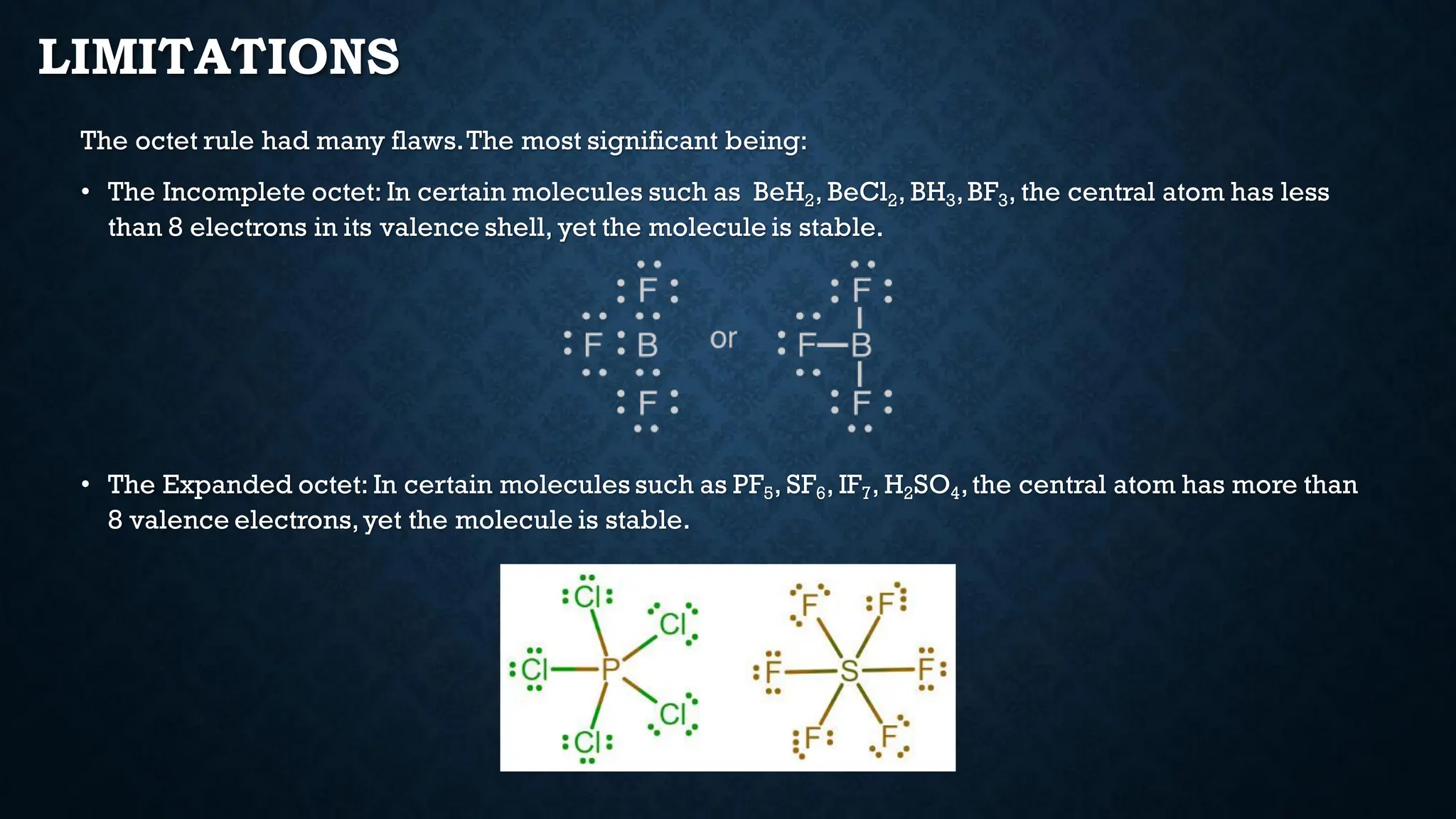
The document discusses the octet rule and its limitations, initially influenced by Richard Abegg's rule which noted a commonality in valence electron configurations. It highlights the breakthroughs by Kossel and Lewis in 1916 that shaped modern chemistry, detailing the octet rule's role in chemical bonding and stability. However, it also points out significant limitations such as incomplete and expanded octets, and the eventual challenges posed by quantum mechanics to traditional theories of bonding.