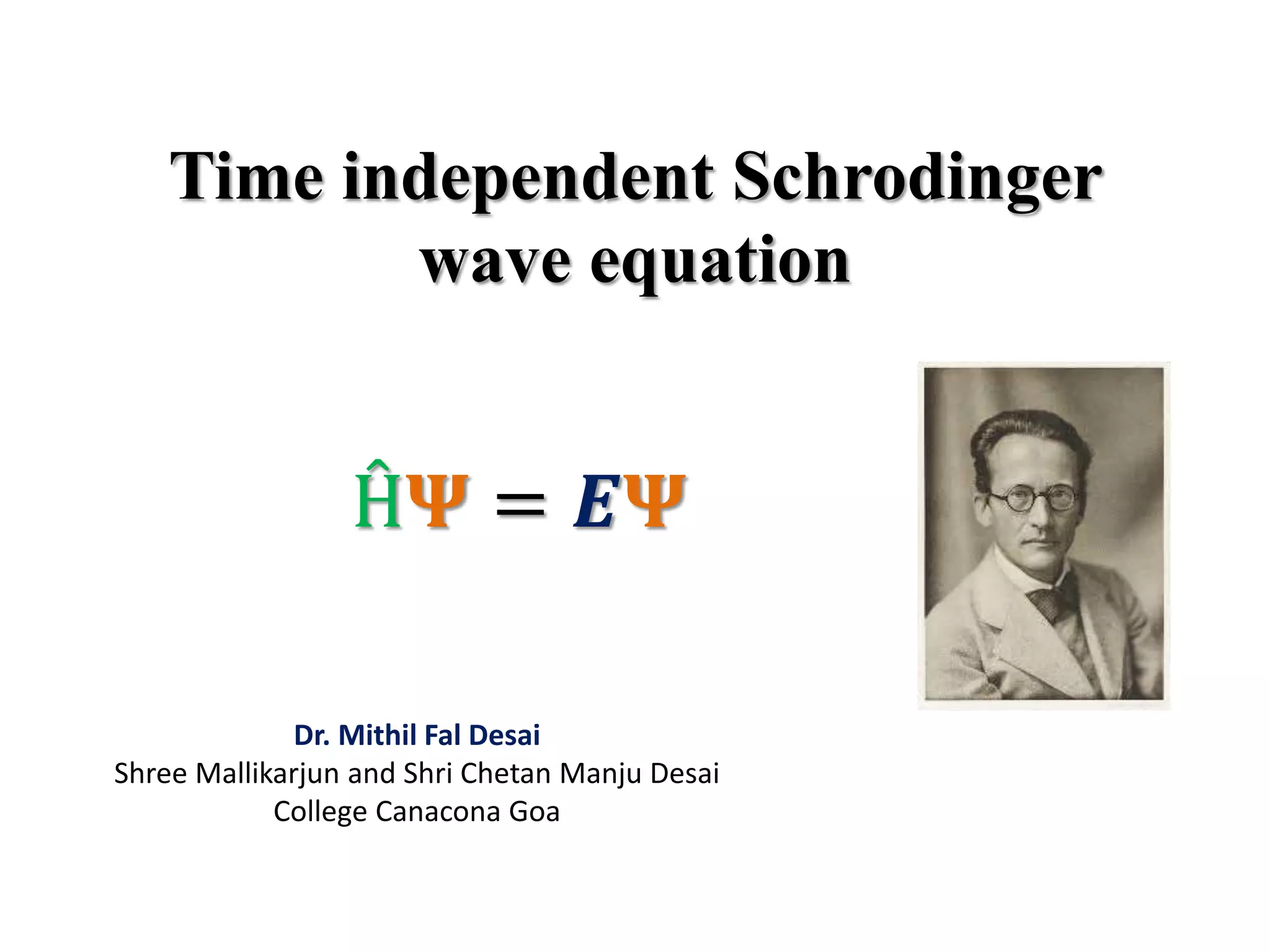
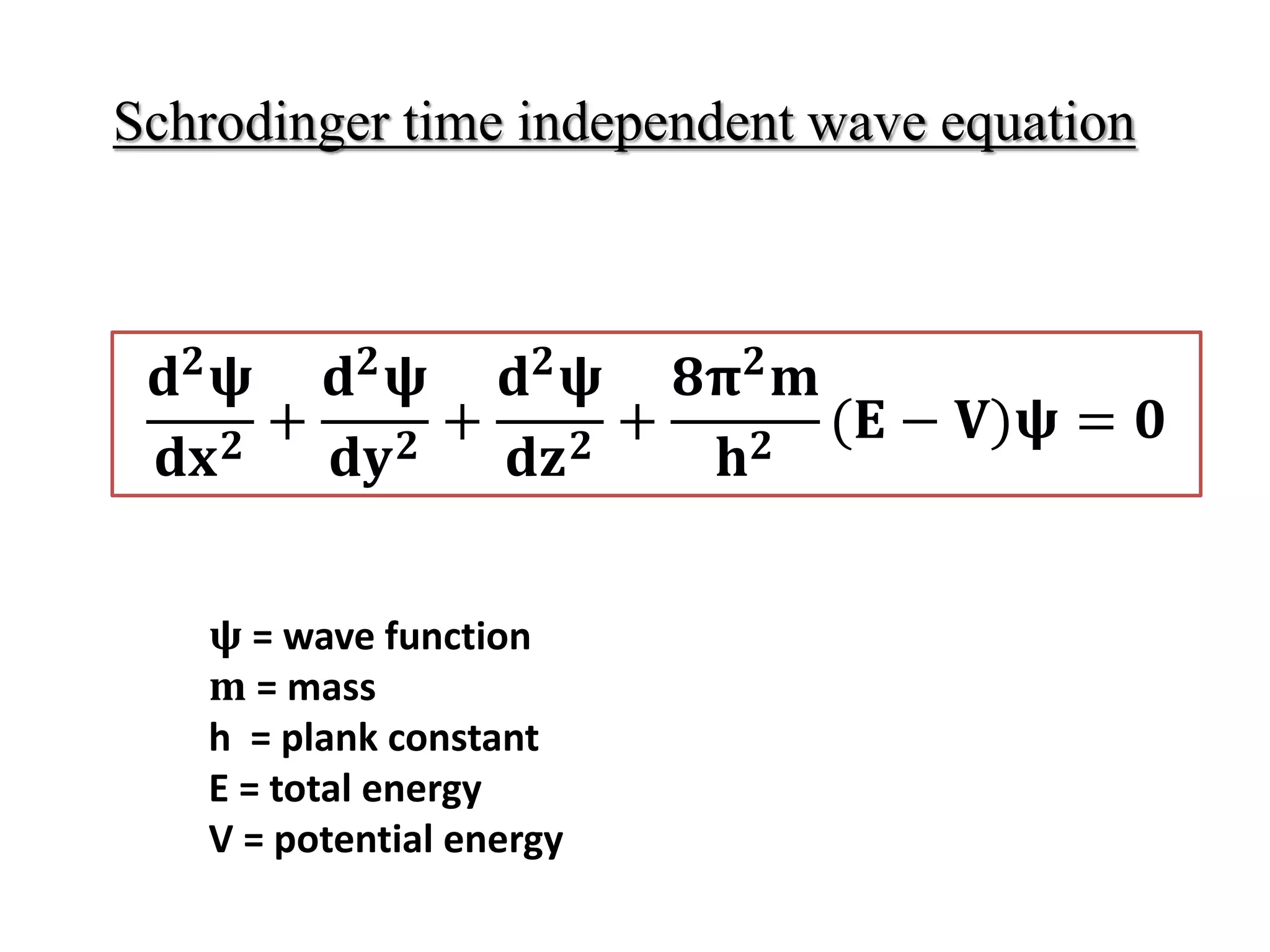
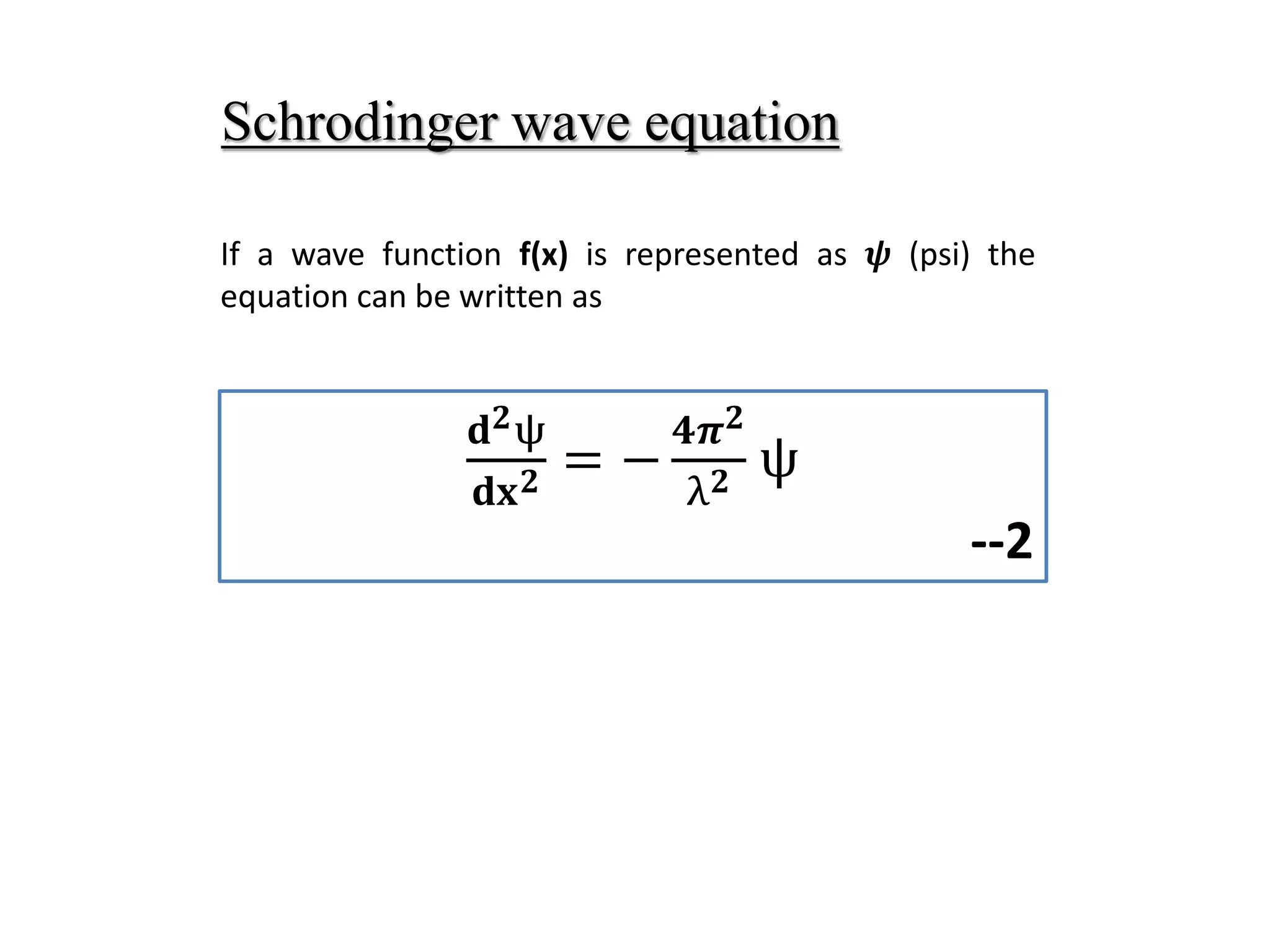
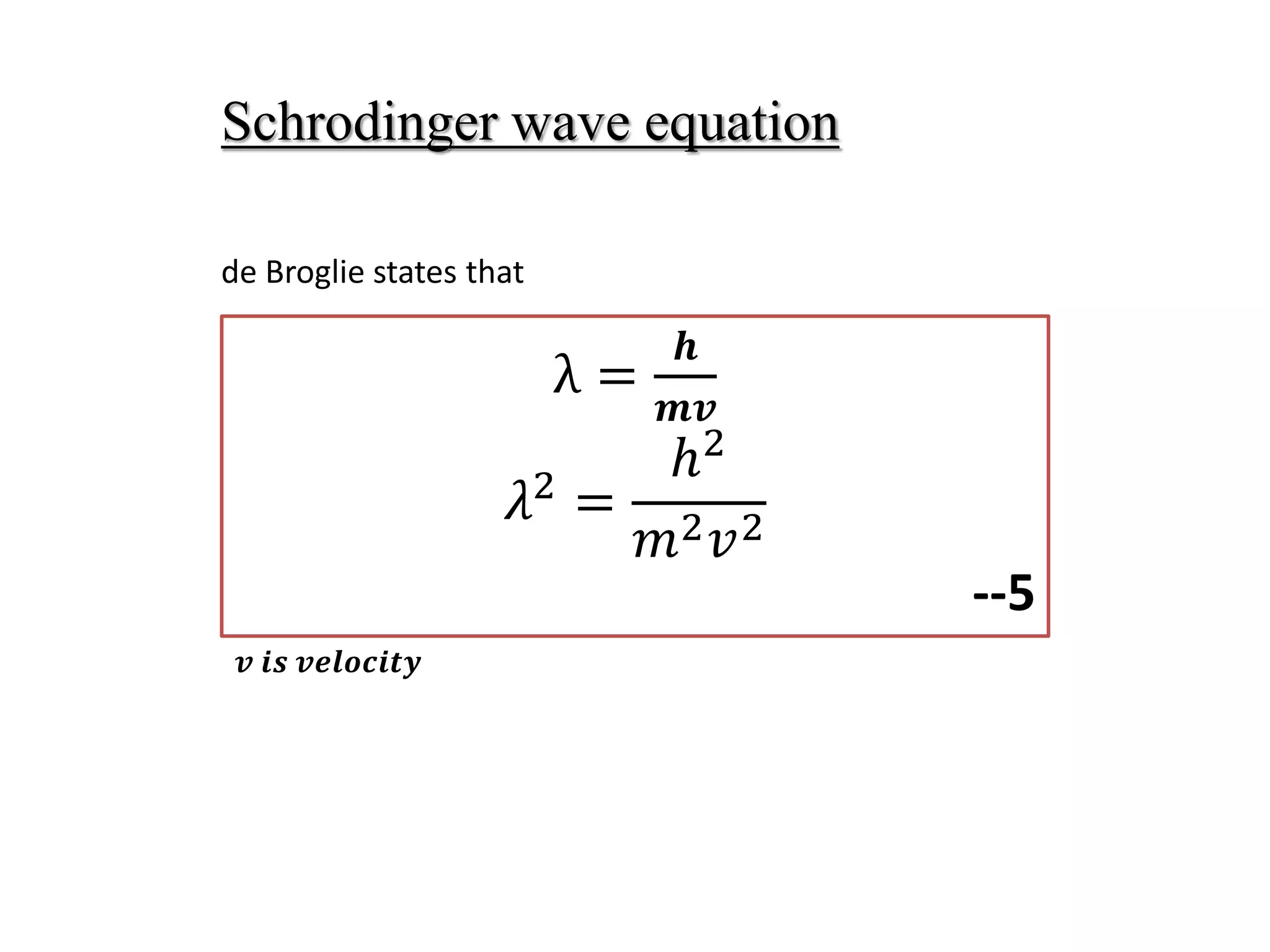
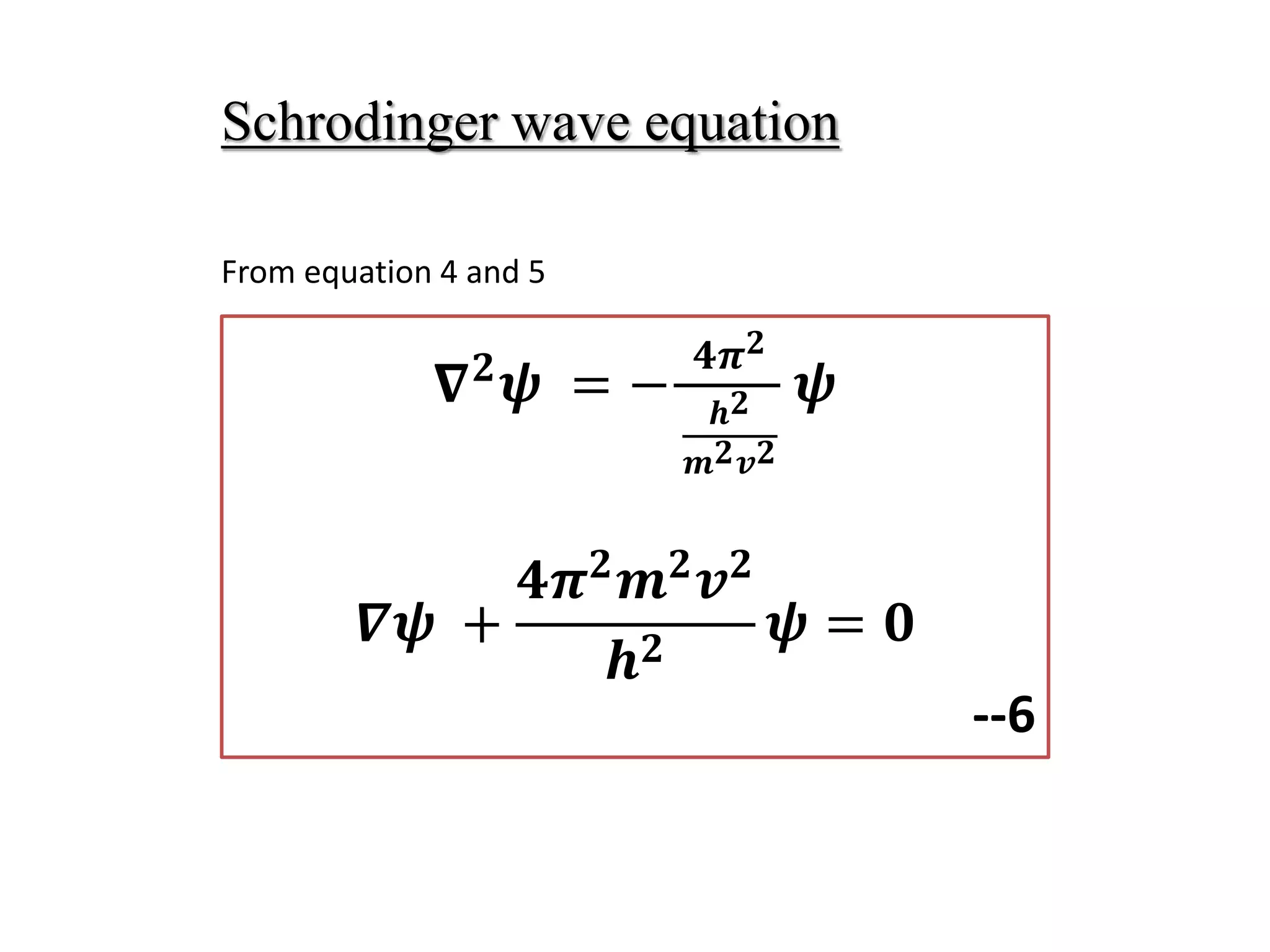
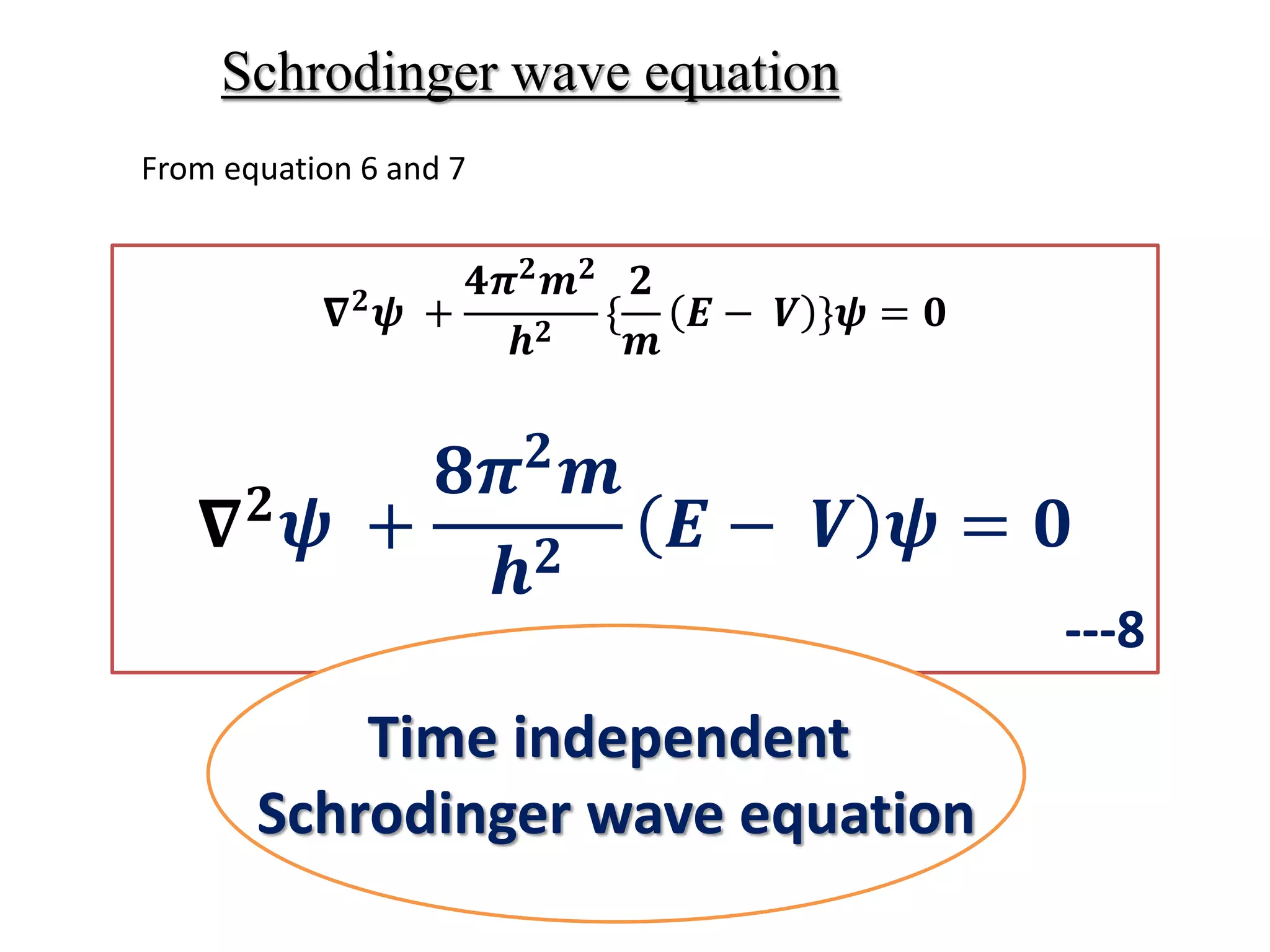
The document summarizes the time-independent Schrodinger wave equation, which describes standing waves as a function and can be written as:
∇2ψ + 8π2m/h2(E - V)ψ = 0
Where ψ is the wave function, ∇2 is the Laplacian operator, m is mass, h is Planck's constant, E is the total energy, and V is the potential energy. This equation describes standing waves in three dimensions using coordinates x, y, and z.