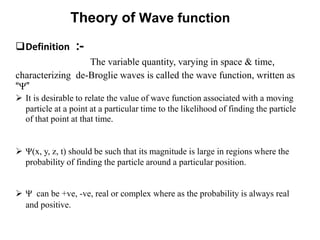
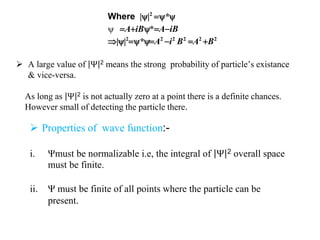
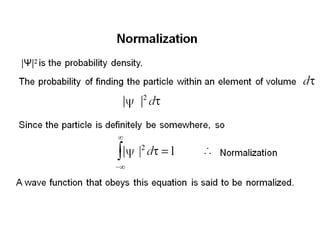
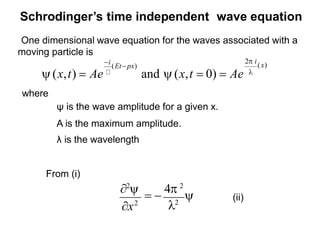
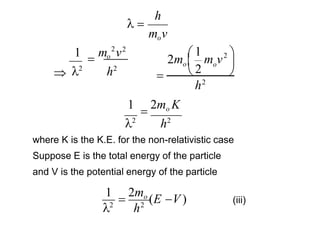
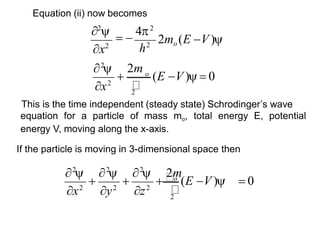
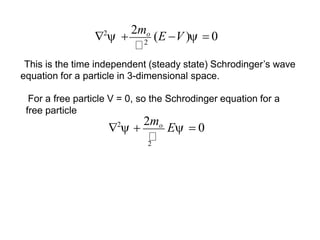
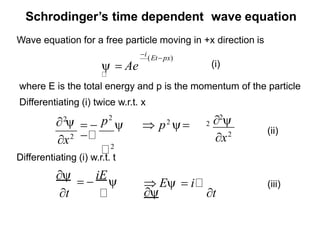
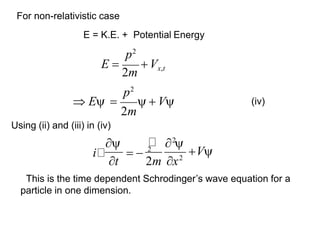
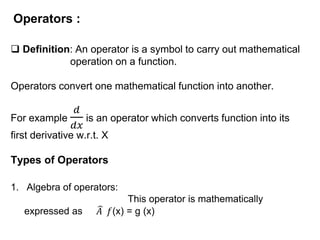
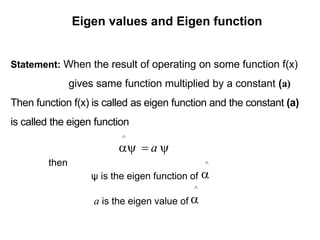
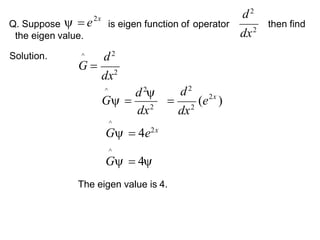
Classical mechanics failed to explain certain phenomena observed at the microscopic level like black body radiation and the photoelectric effect. This led to the development of quantum mechanics, with key aspects being the wave function Ψ, Schrodinger's time-independent and time-dependent wave equations, and operators like differentiation that act on wave functions to produce other wave functions. The wave function Ψ relates to the probability of finding a particle, with |Ψ|2 representing the probability.