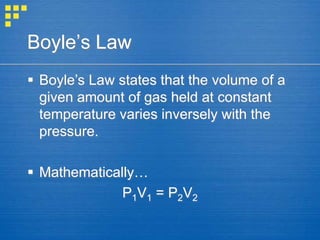
The document discusses the kinetic molecular theory, which models the behavior of gases. Some key ideas are that gas particles are in constant, random motion and collide elastically with each other and container walls. Gas particles are separated by relatively large distances. Gas pressure results from collisions with container walls. Temperature is defined as the average kinetic energy of gas particles. The behavior of gases depends on number of particles, pressure, temperature, and volume. Several gas laws relating these factors are also introduced.