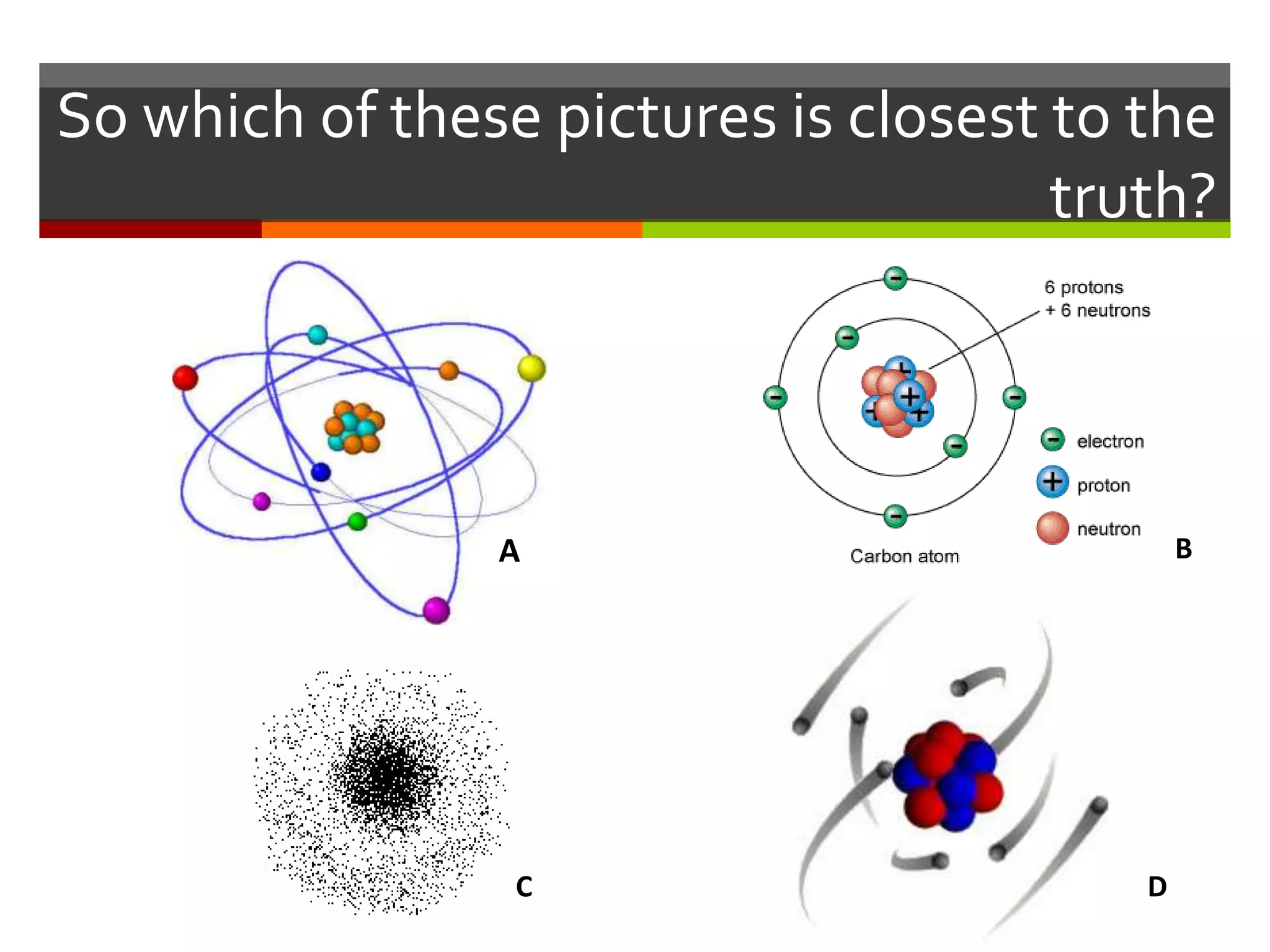
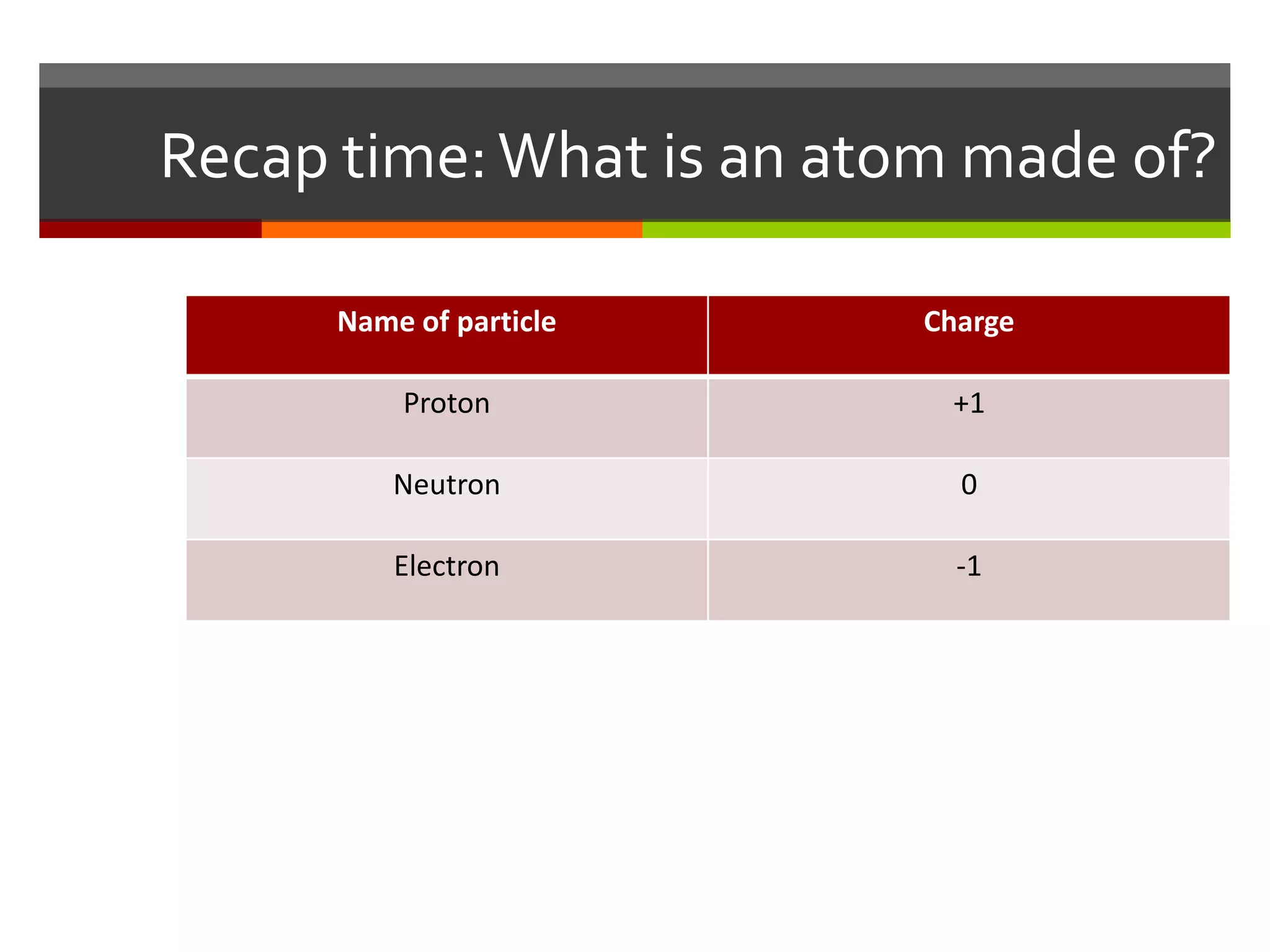
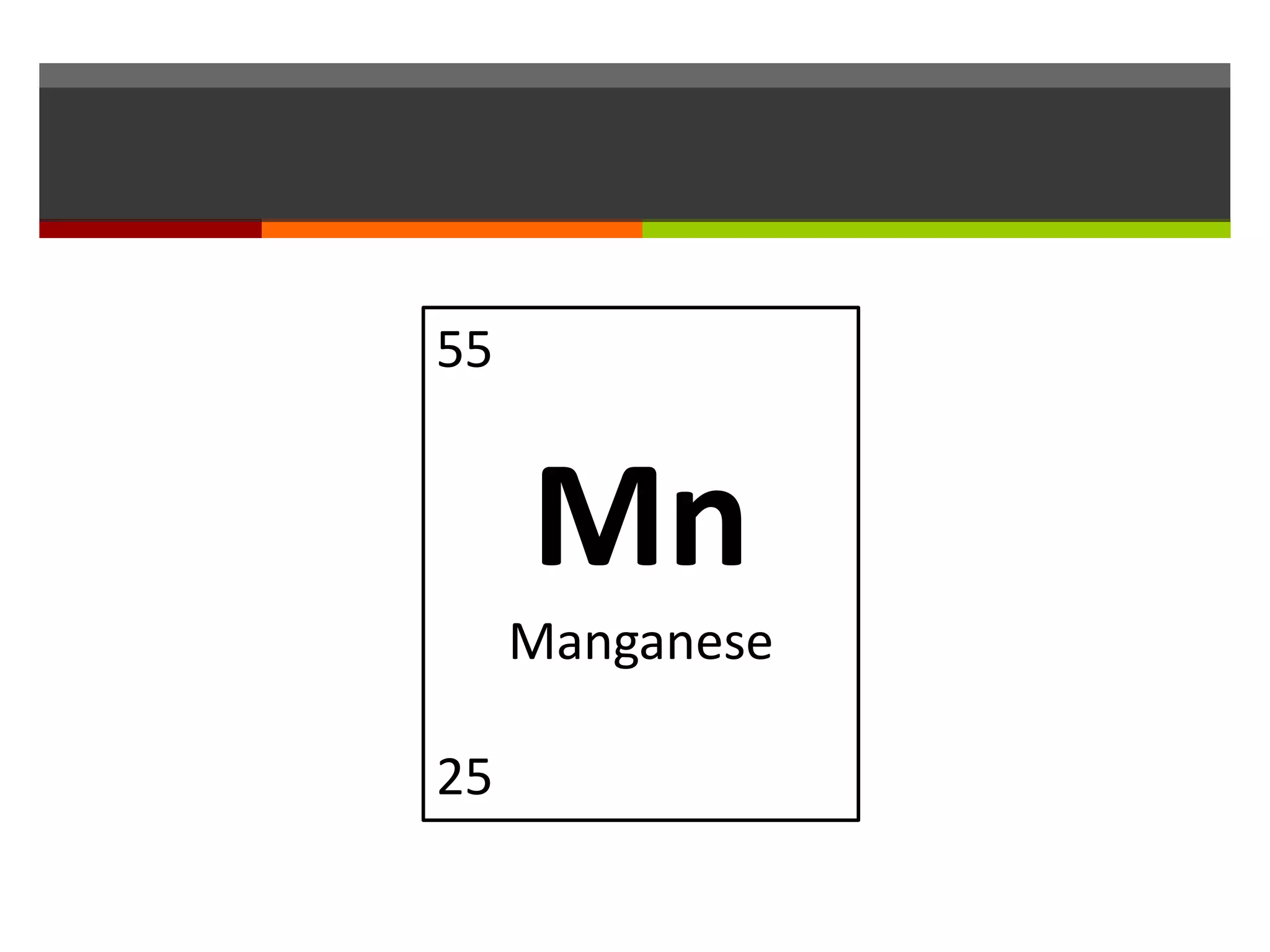
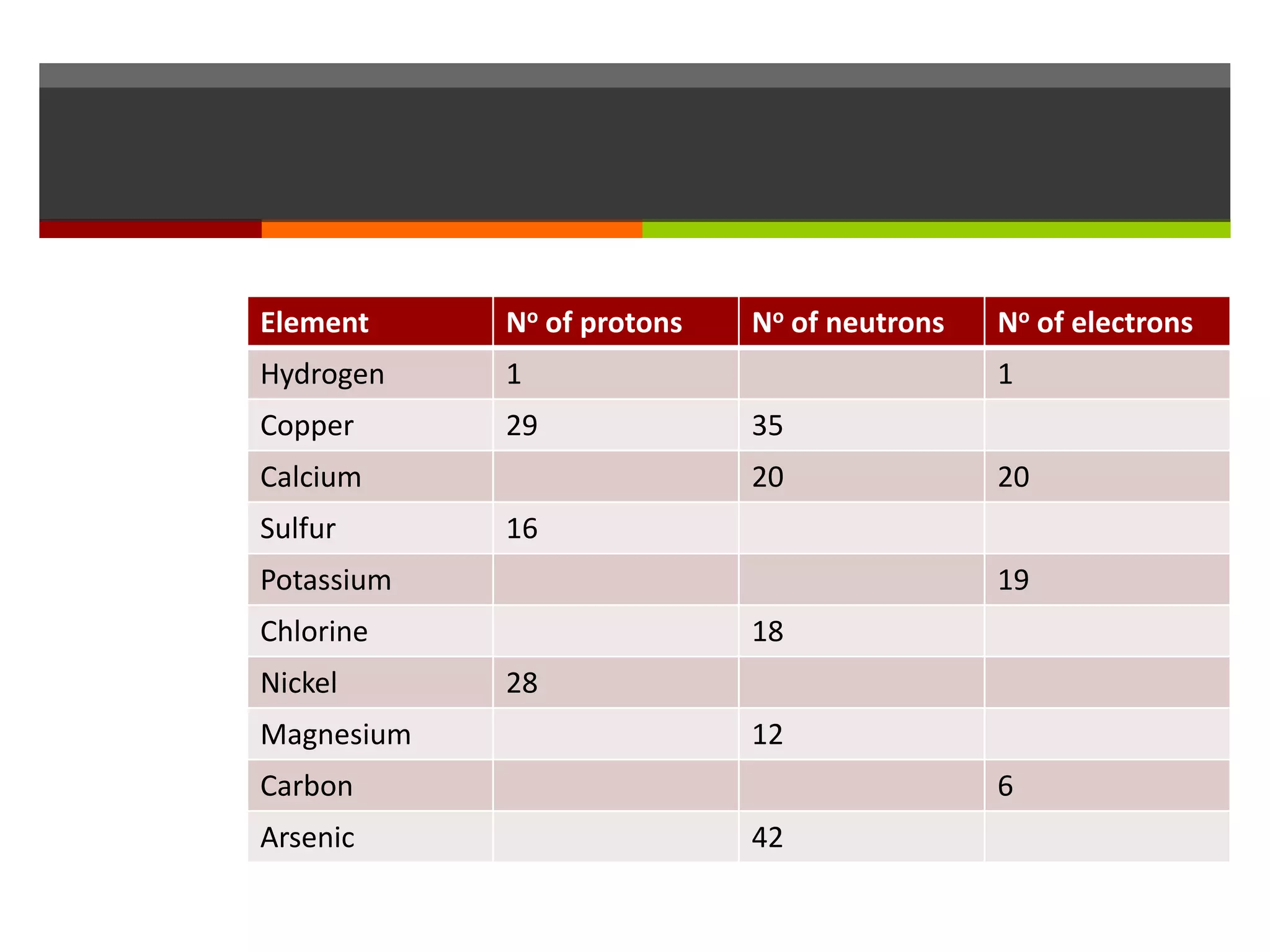
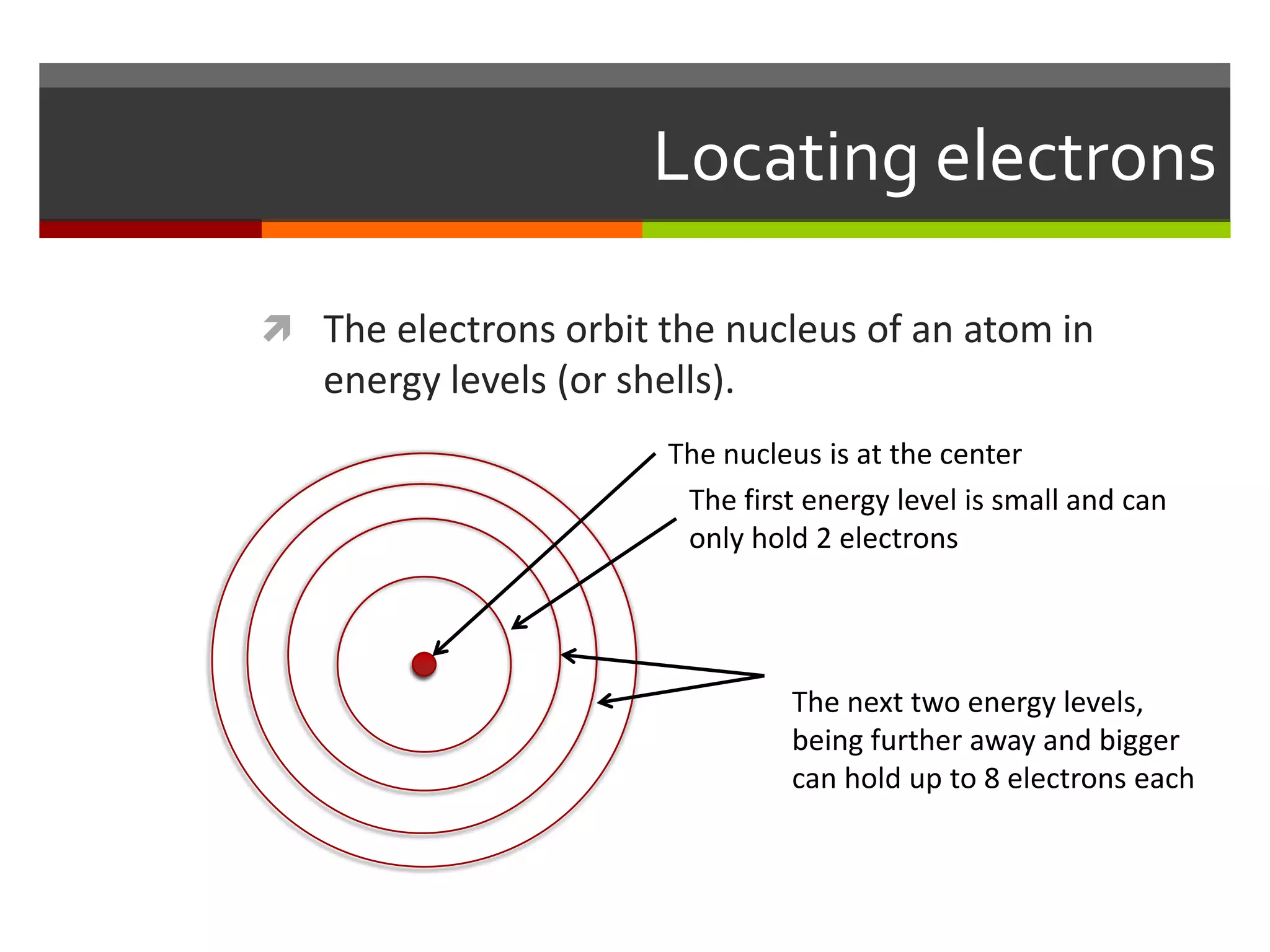
The document discusses atoms and their structure. It explains that atoms are made up of protons, neutrons, and electrons. Protons and neutrons are located in the nucleus at the center of the atom, while electrons orbit around the nucleus in energy levels. The document also notes that atoms are very small, around 0.00000001mm in diameter, and discusses how scientists have worked to discover the structure of atoms over time.