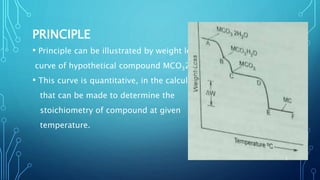
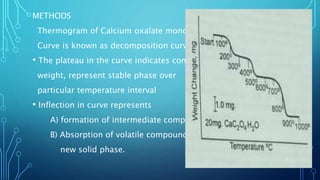
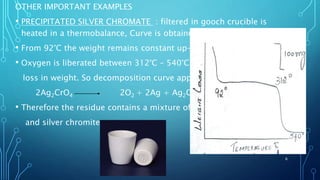
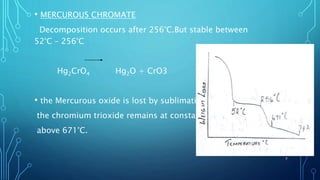
Thermogravimetric analysis (TGA) measures the mass of a substance as the temperature changes. It provides quantitative data on weight changes from thermal transitions like decomposition or evaporation. TGA can be either dynamic, with continuous temperature increase, or isothermal at constant temperature. The technique graphs weight against temperature or time. The data can identify phases and stoichiometries of compounds. Factors like heating rate, atmosphere, and sample properties affect TGA results. It has advantages like minimal sample prep and fast analysis, but data interpretation can be complex.