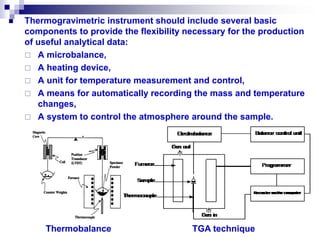
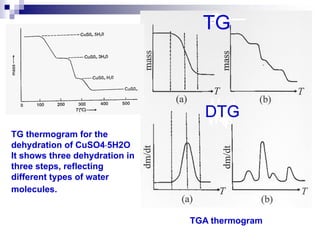
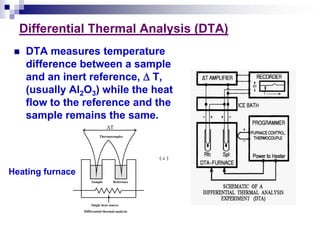
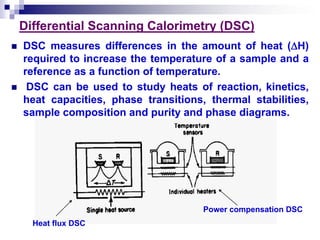
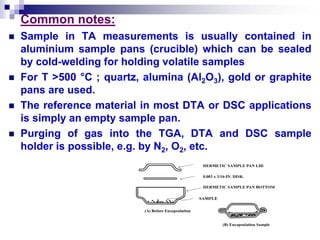
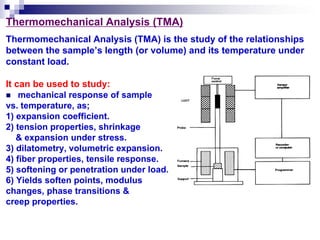
Thermal analysis techniques measure physical and chemical properties of substances as a function of temperature. Common techniques include thermogravimetric analysis (TGA), differential thermal analysis (DTA), and differential scanning calorimetry (DSC). TGA measures weight changes, DTA measures temperature differences between a sample and reference, and DSC measures heat flows. These techniques are useful for characterizing materials across many industries including polymers, chemicals, pharmaceuticals, foods, and metals. They provide information about phase transitions, decomposition reactions, purity, and more.