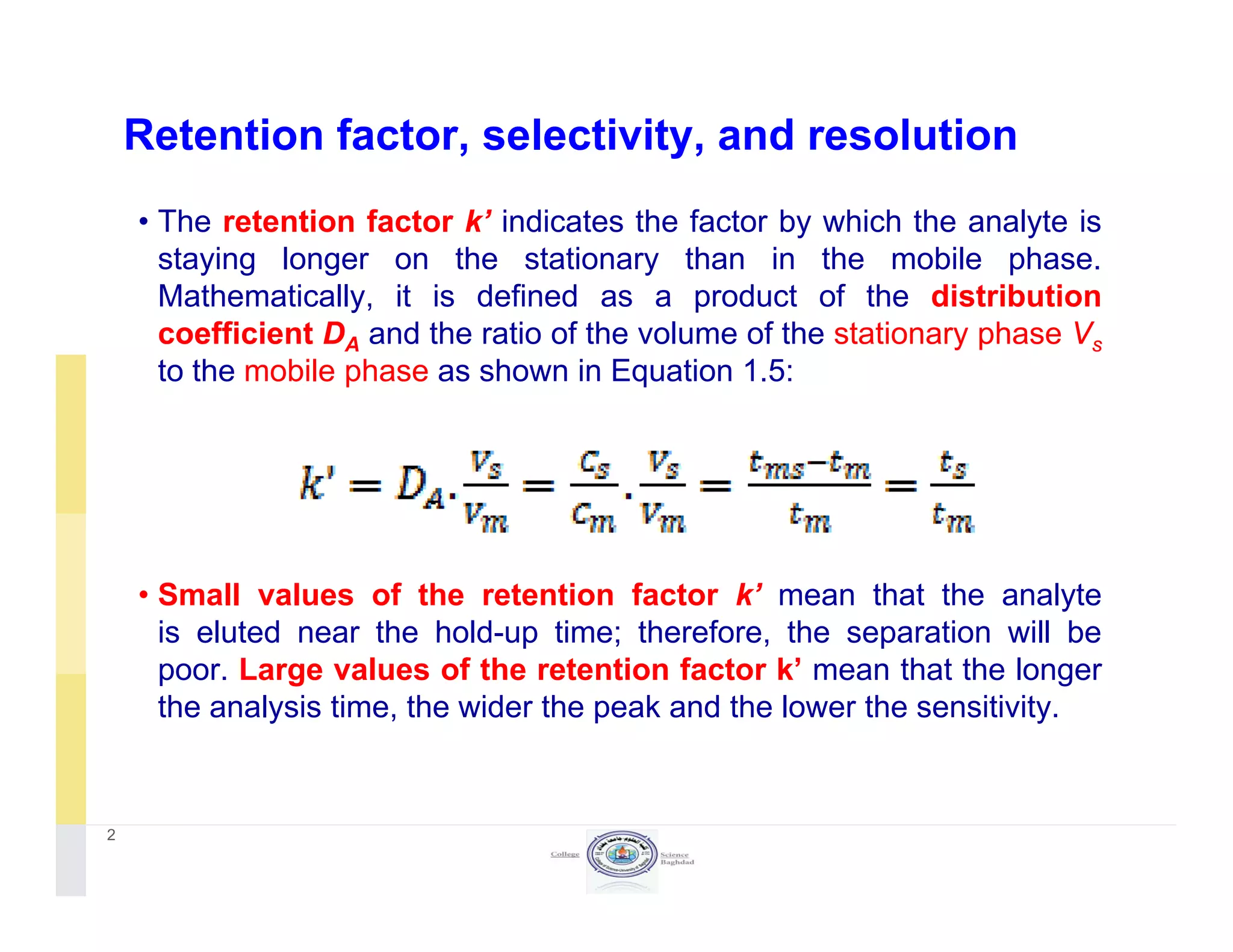
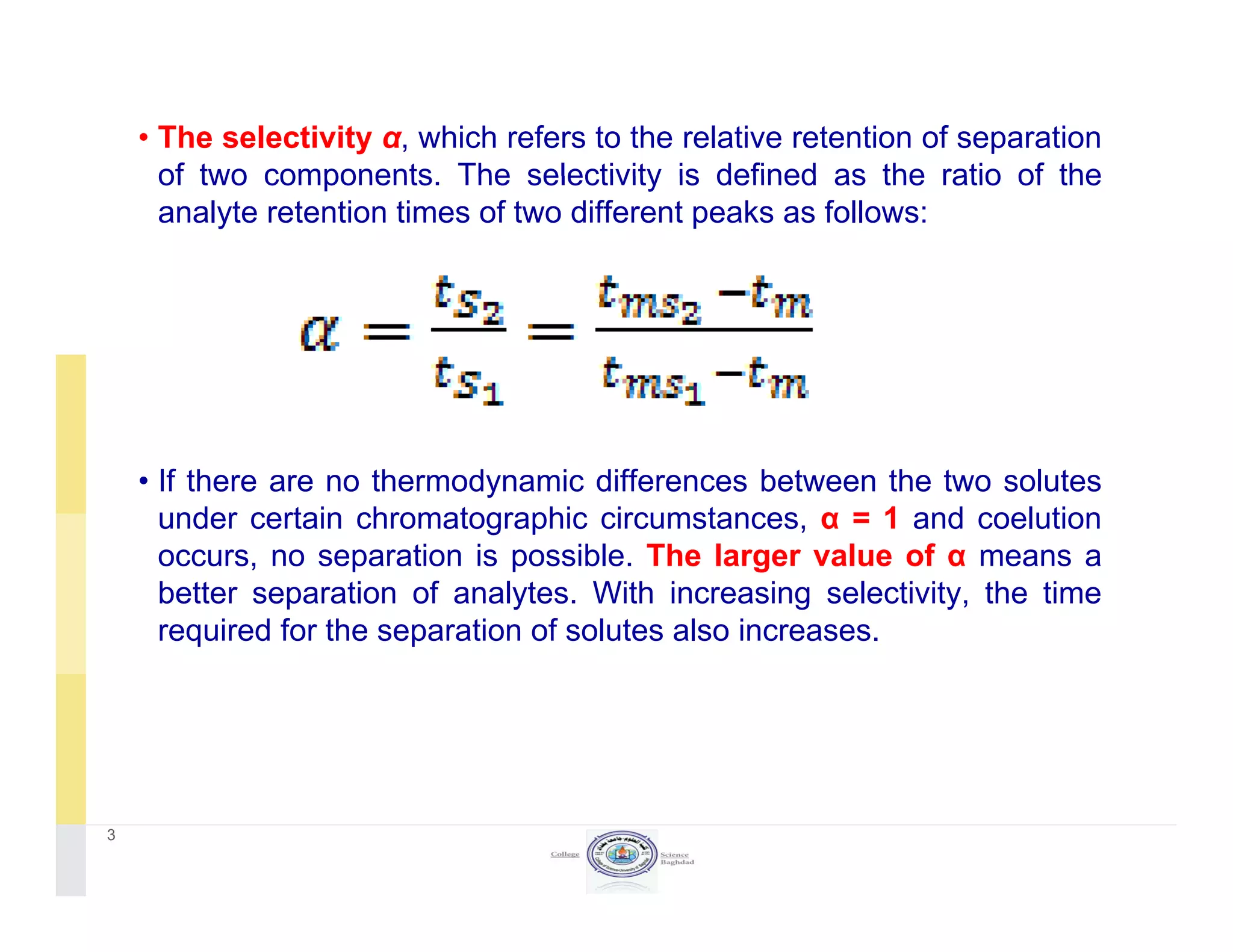
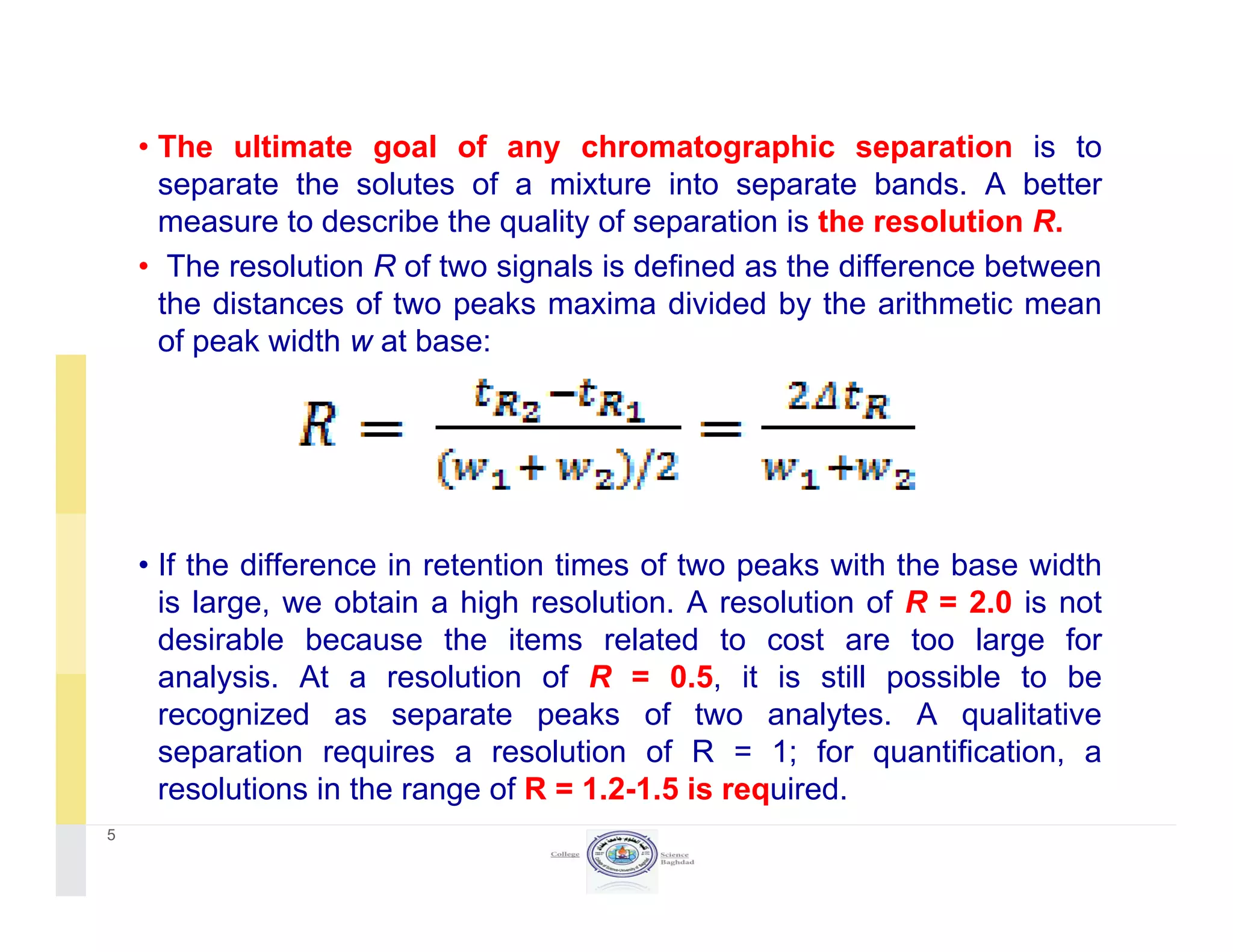
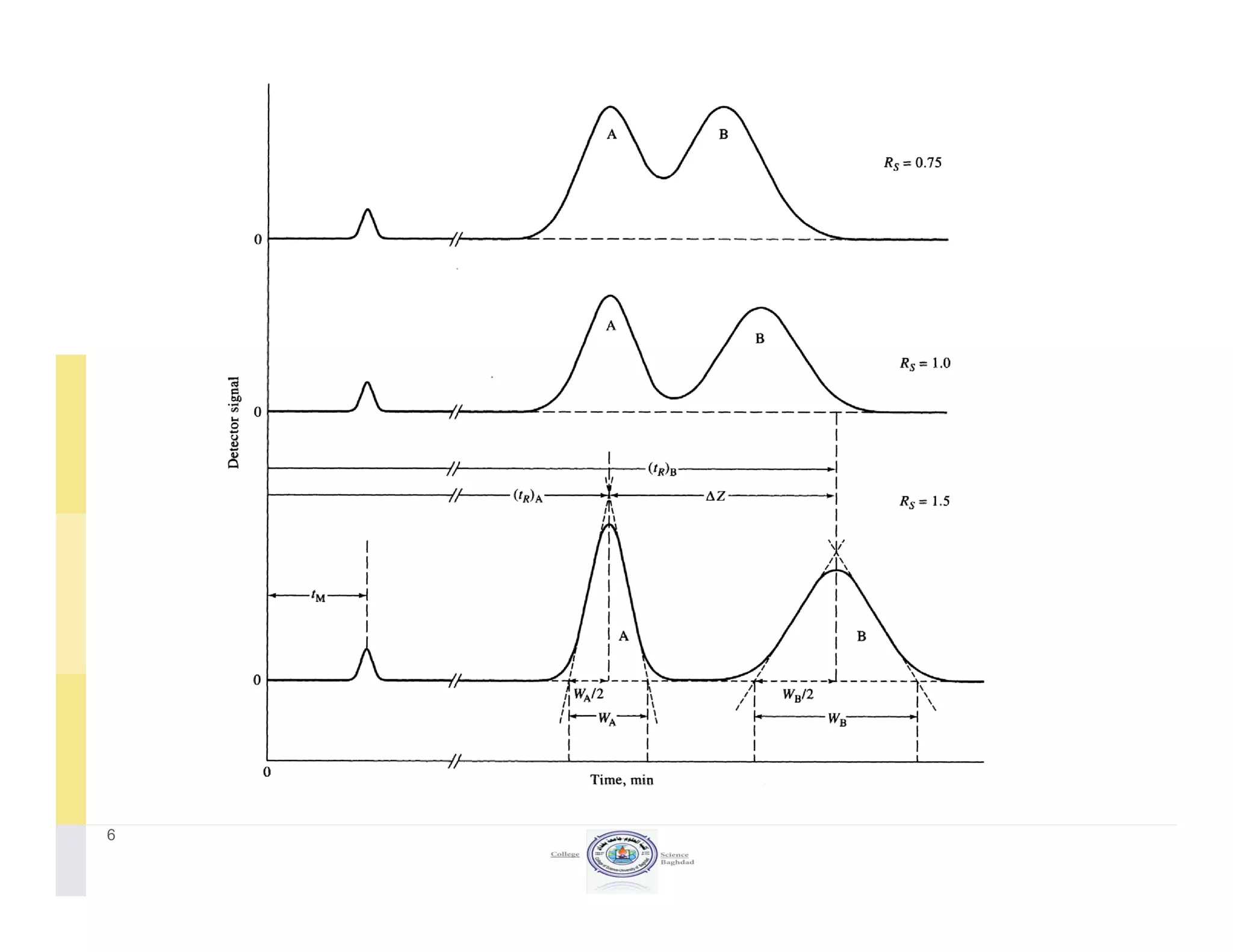
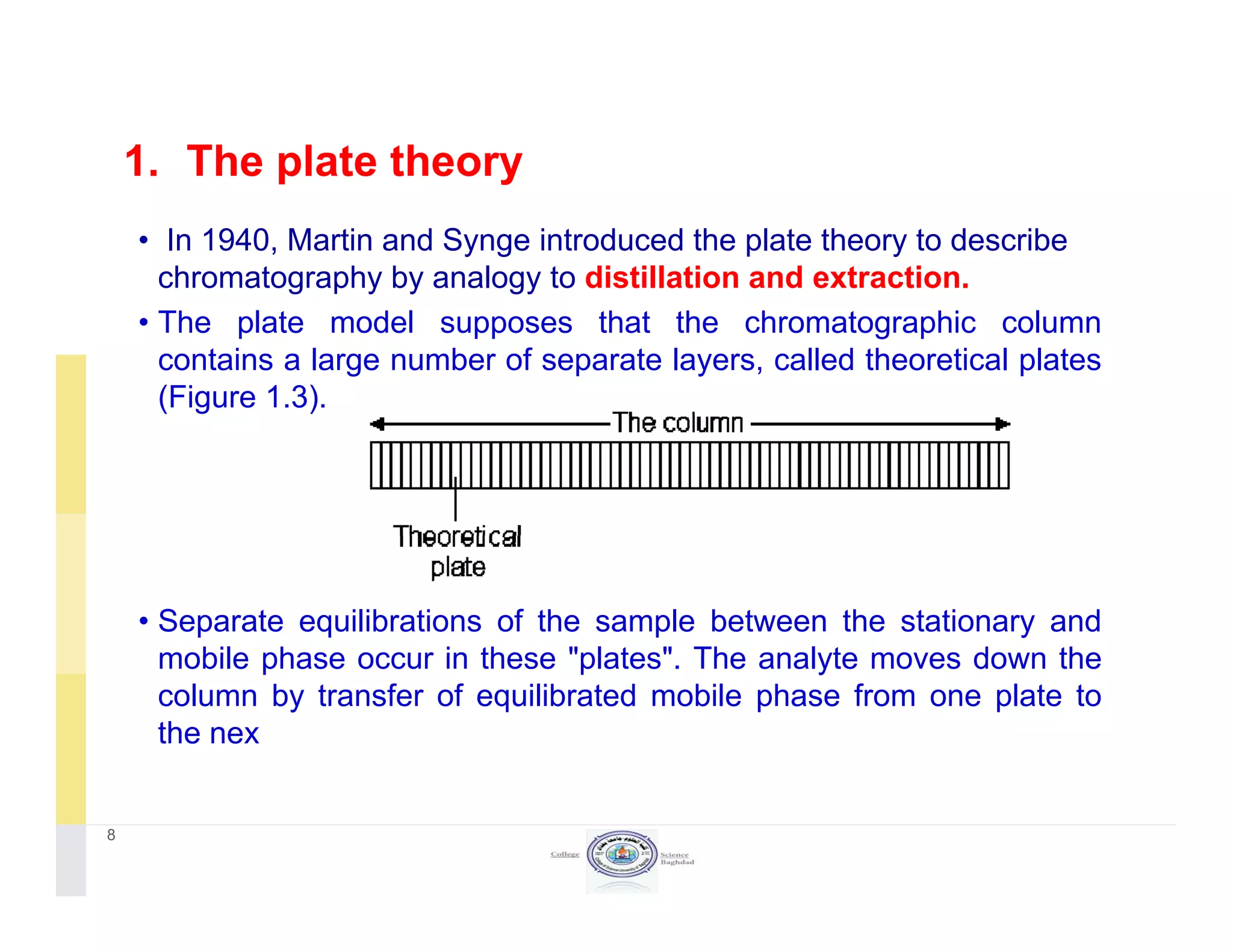
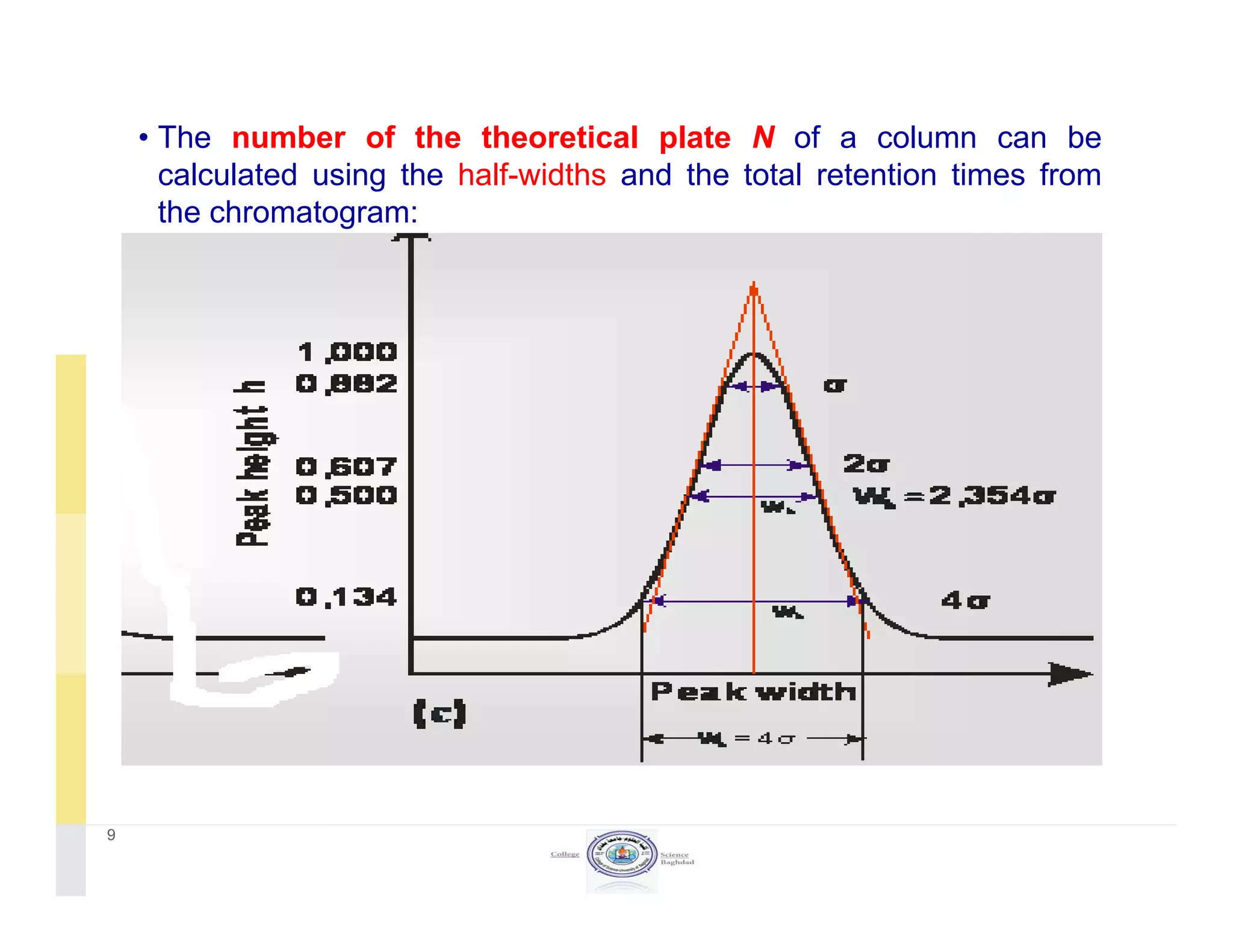
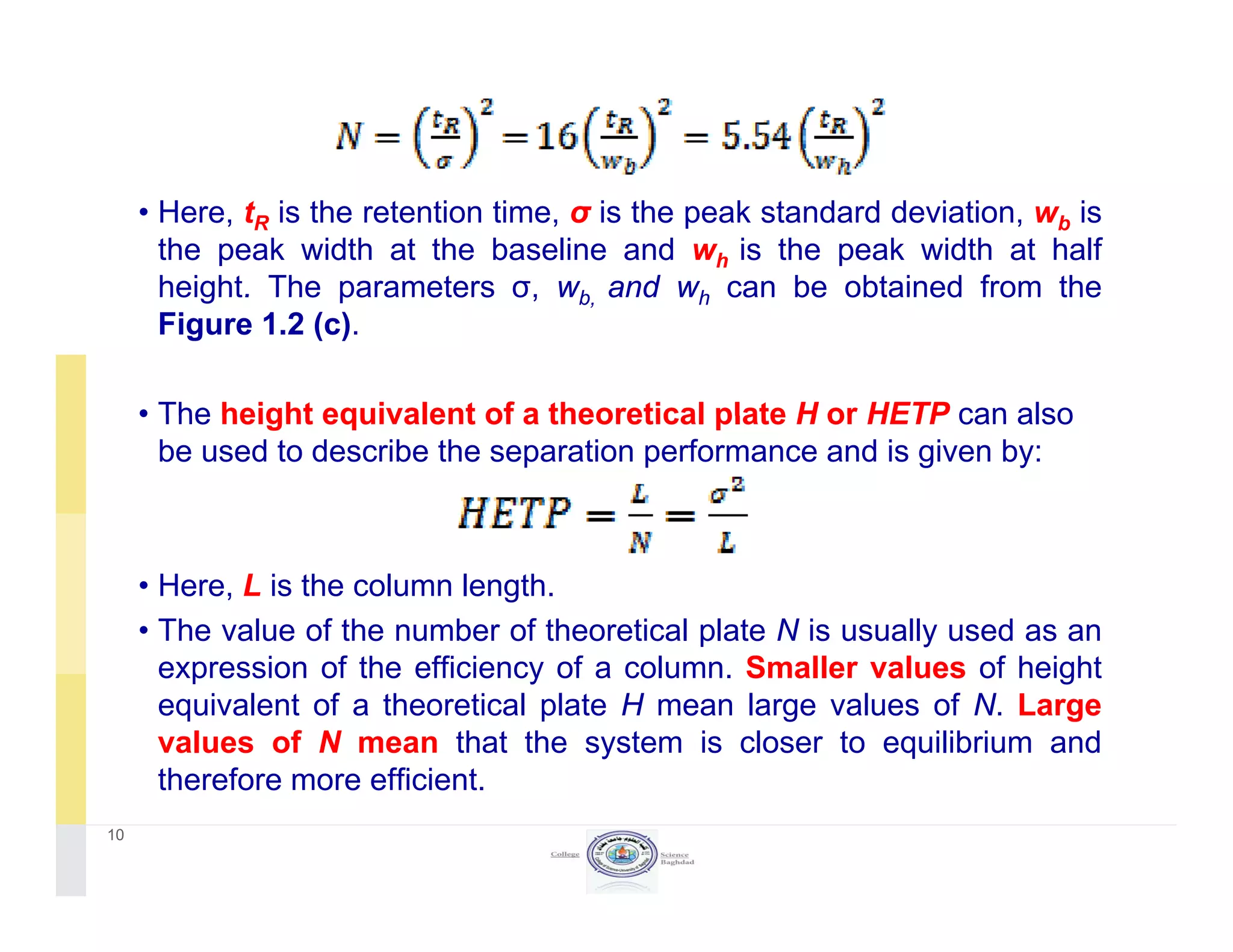
The document discusses key concepts in chromatography, focusing on retention factor, selectivity, and resolution. It explains how these metrics impact the separation quality of analytes in a mixture, emphasizing the importance of achieving high resolution for effective analysis. Additionally, it outlines the plate theory and dynamic theory that underlie chromatographic processes.