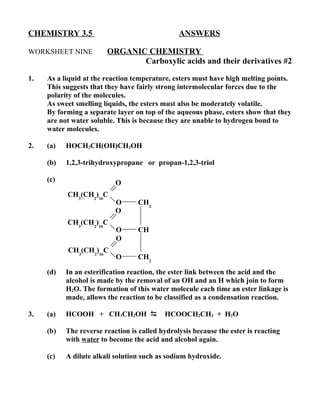
1) Esters have fairly strong intermolecular forces due to polarity and form separate layers from water since they cannot hydrogen bond with water molecules.
2) Amides are usually made by reaction of acyl halides with ammonia.
3) Amides have high boiling points and are soluble in water because they can hydrogen bond to themselves and water molecules.