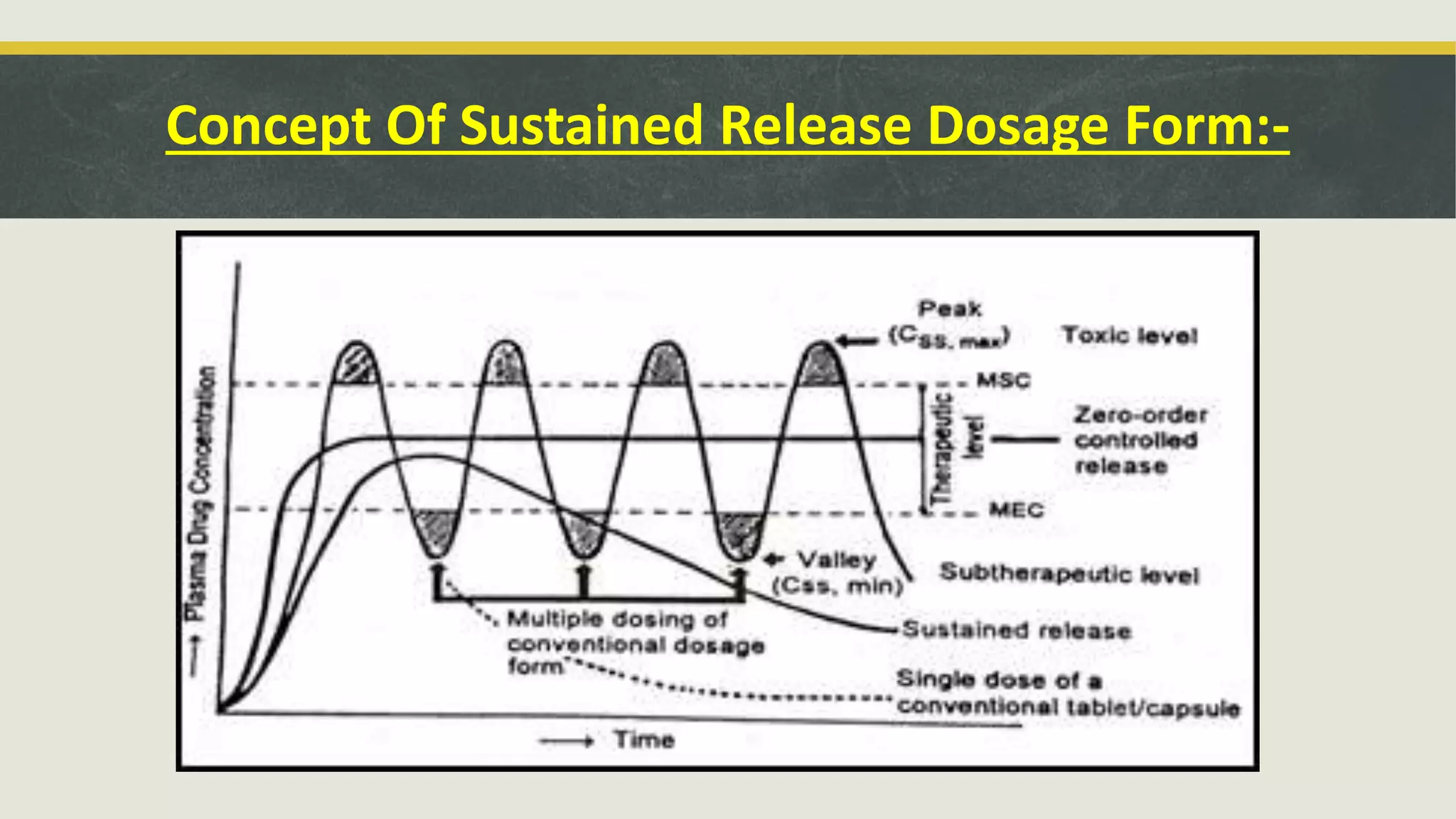
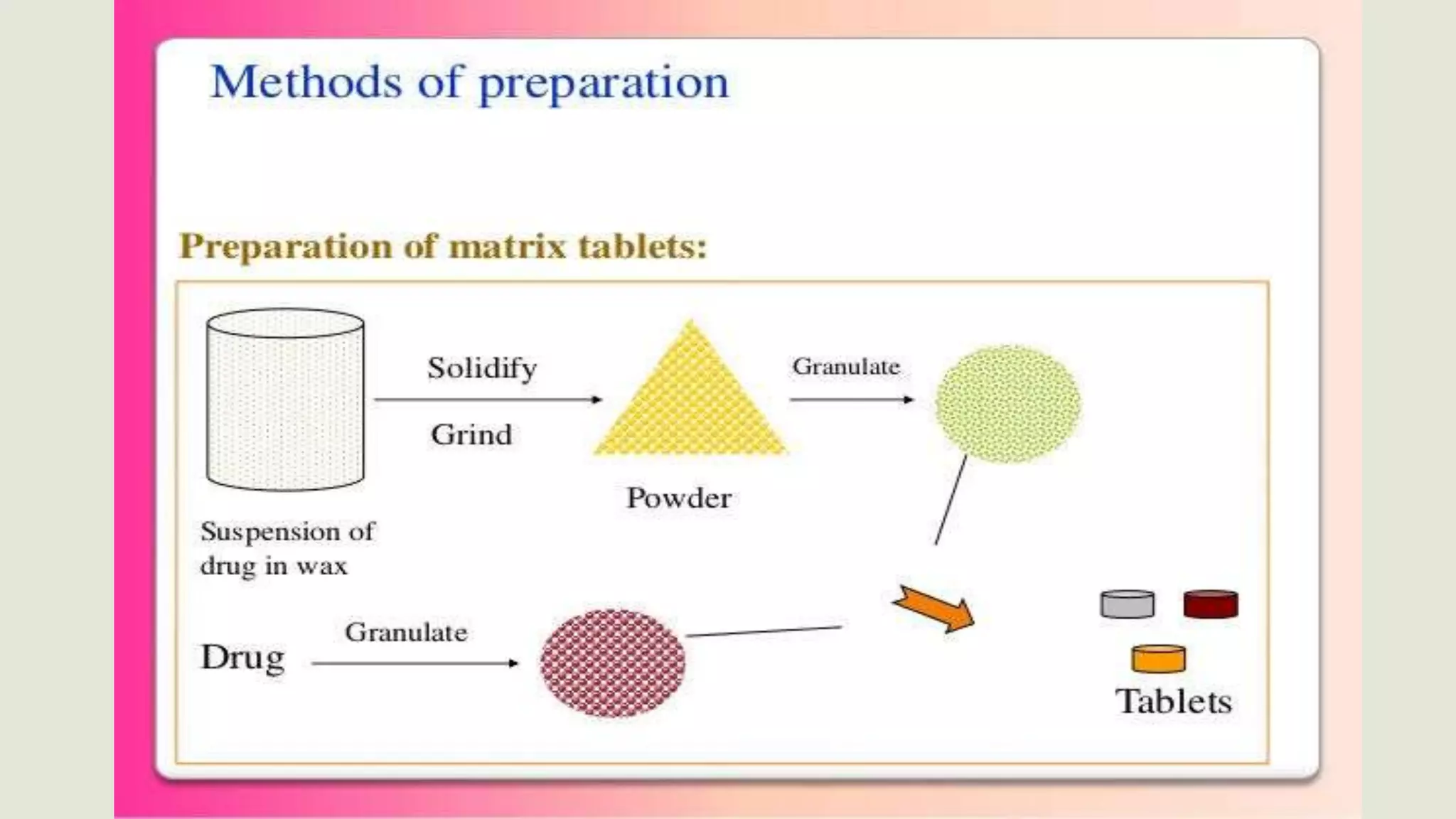
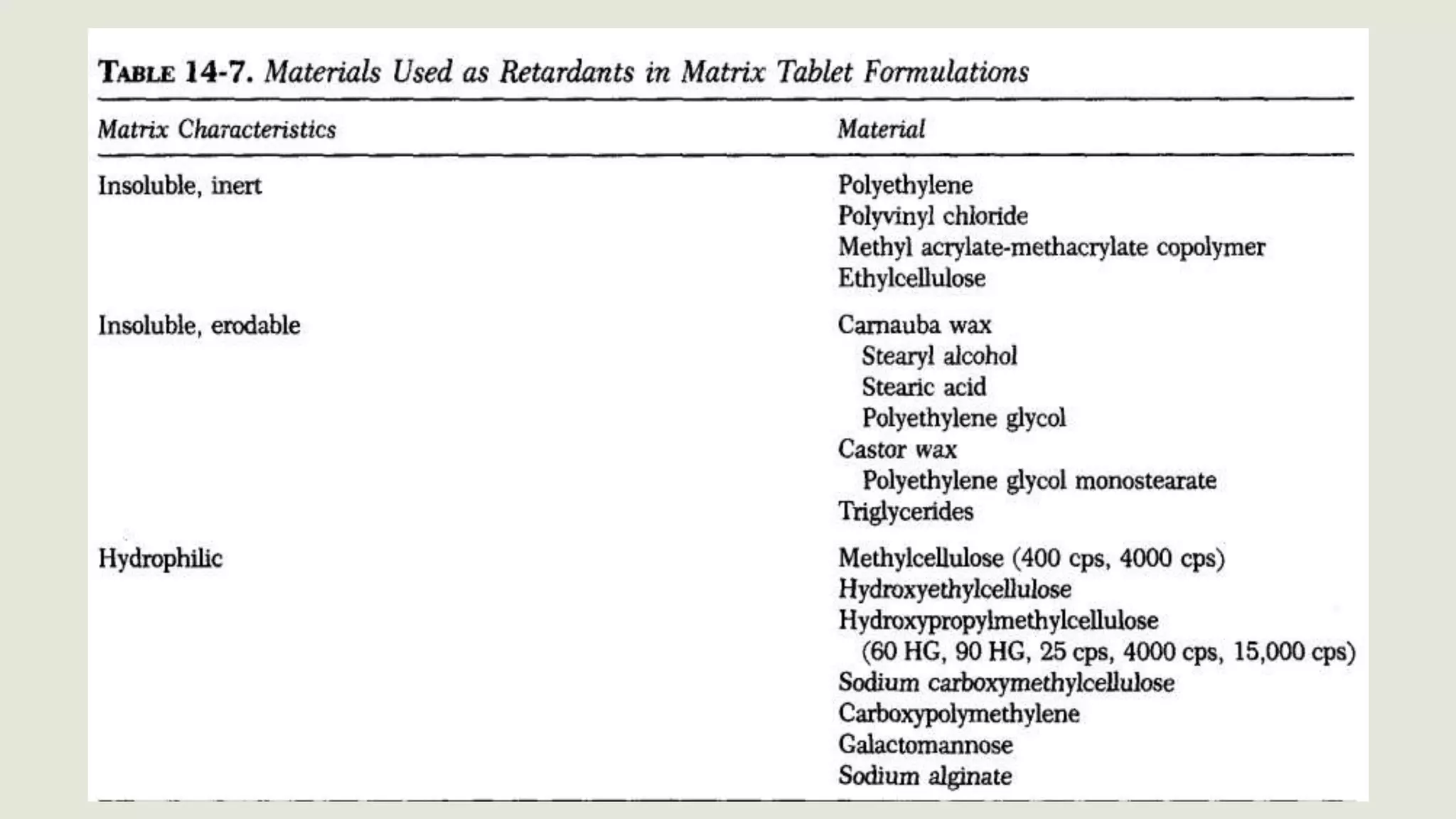
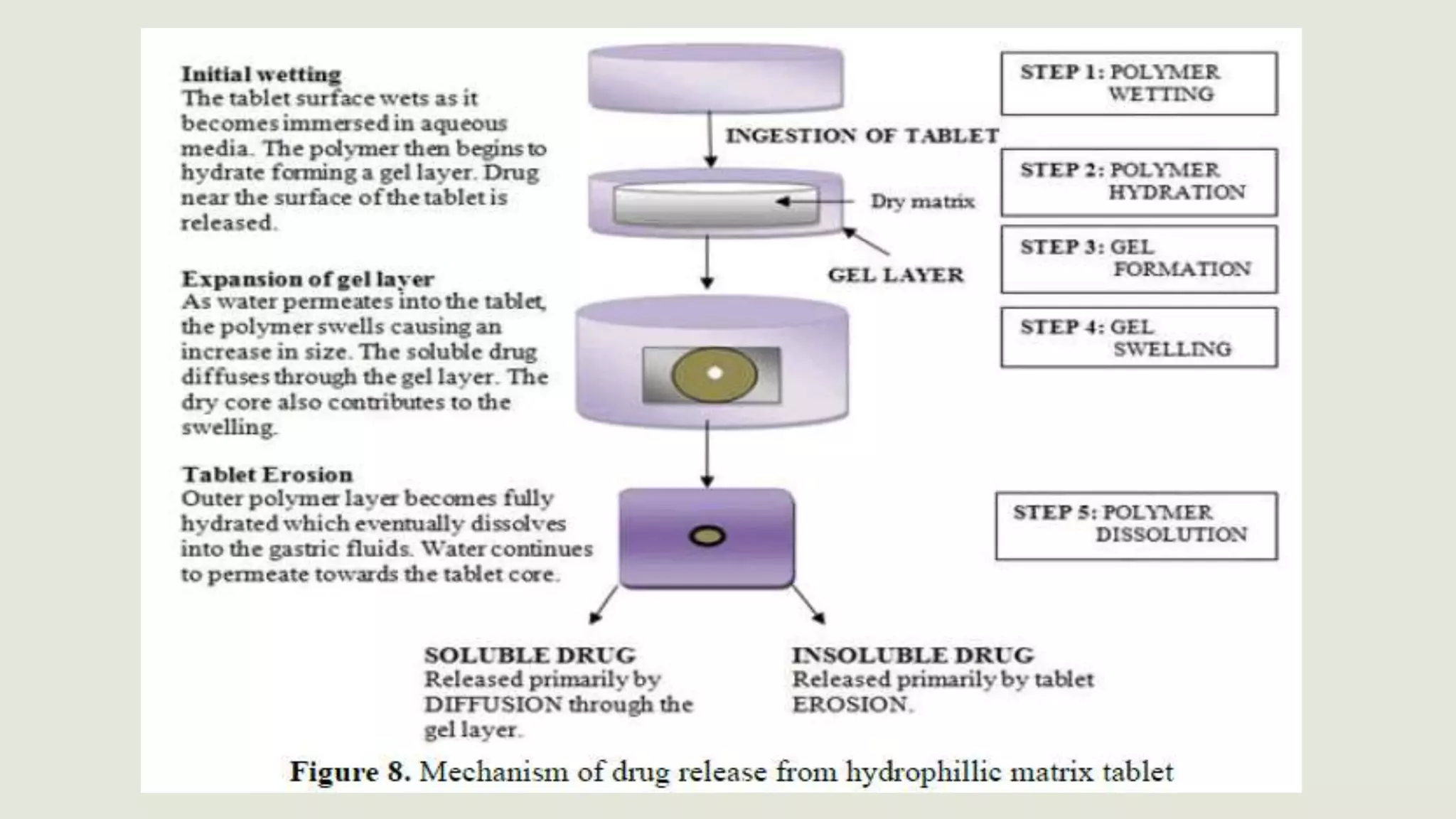
This document discusses sustained release oral dosage forms. It defines sustained release as achieving a steady blood or tissue level of a drug over an extended period of time. This is beneficial for drugs with short half-lives that require repeated dosing. The document outlines criteria for drugs to be formulated as sustained release, including desirable half-life and absorption characteristics. Advantages include improved patient compliance from less frequent dosing and better drug utilization. Matrix tablets are discussed as a common sustained release formulation where the drug is uniformly dispersed within a polymeric matrix to control release rate.