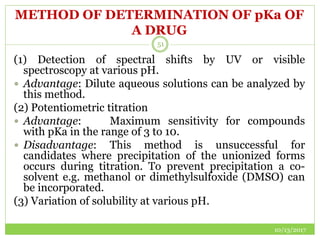
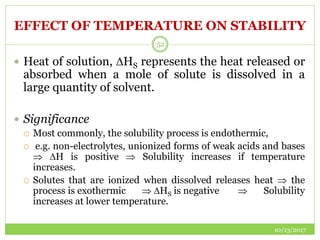
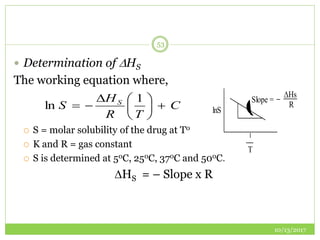
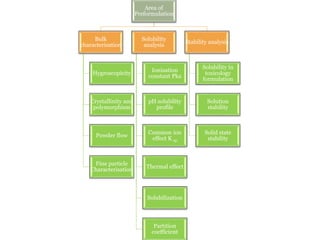
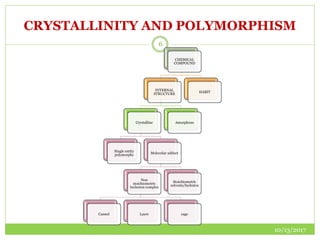
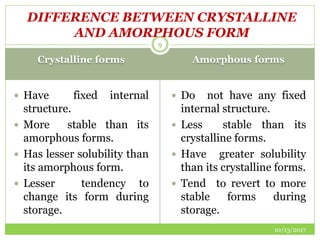
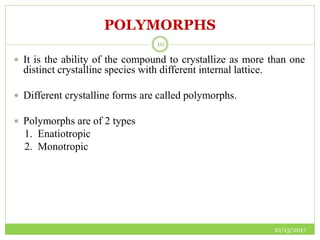
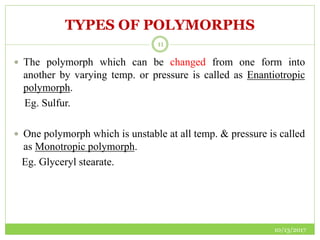
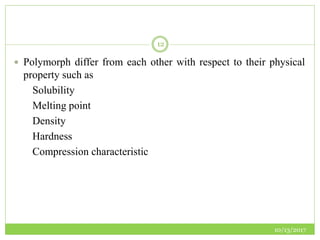
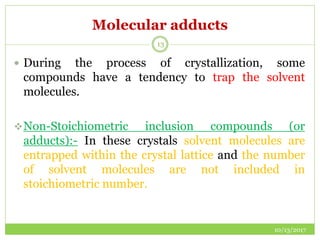
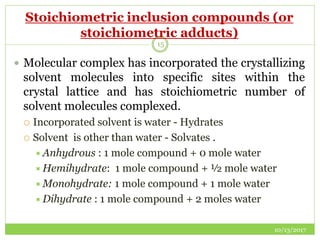
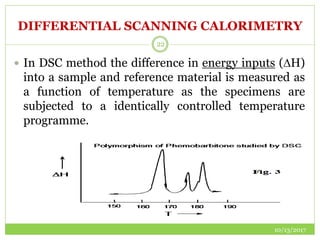
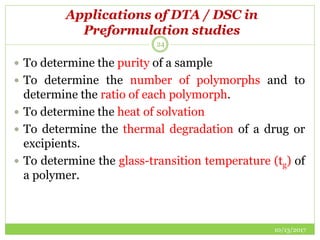
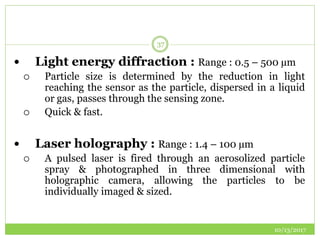
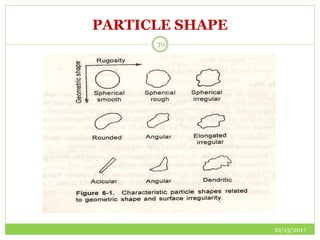
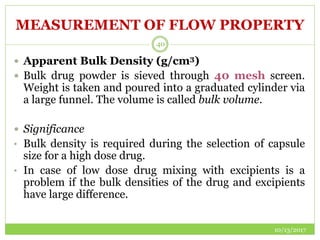
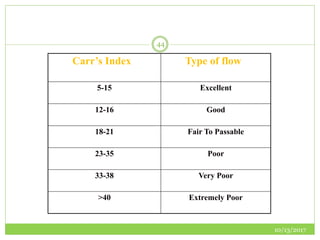
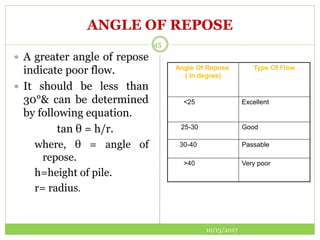
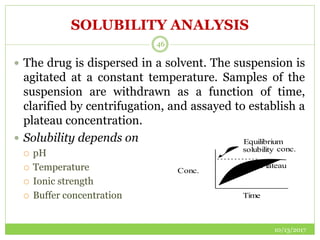
This document discusses pre-formulation studies, which involve investigating the physical and chemical properties of drug substances alone and when combined with excipients. Some key areas covered include polymorphism, hygroscopicity, particle size characterization, and solubility analysis. Thermal analysis techniques like DSC and XRD are described as useful for characterizing polymorphs. The importance of solubility studies at various pH levels and temperatures is highlighted for developing oral dosage forms with appropriate dissolution profiles.



















![IONIZATION CONSTANT (pKa)
10/13/2017
50
When a weakly acidic or basic drug partially ionizes in GI
fluid, generally, the unionized molecules are absorbed
quickly.
Can be calculated by Henderson Hasselbach equation-
For acidic drugs….pH= pKa+ log [ionized drug]
[unionized drug]
For basic drugs….pH= pKa+ log[unionized drug]
[ionized drug]](https://image.slidesharecdn.com/preformulation-171013052101/85/Pre-Formulation-50-320.jpg)