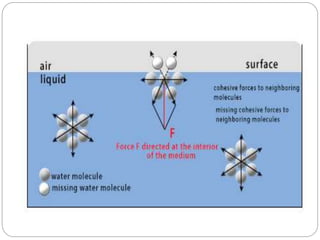
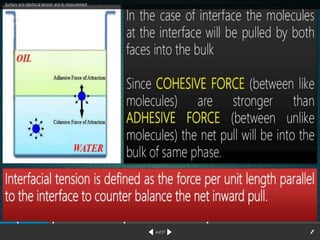
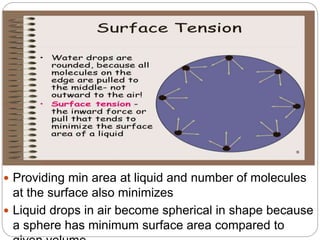
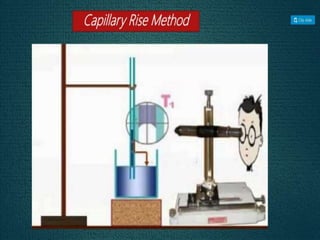
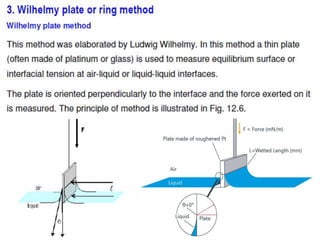
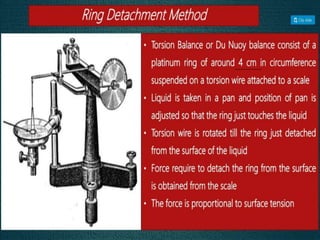
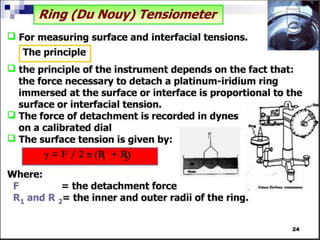
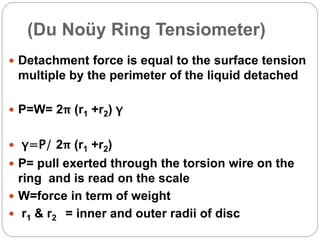
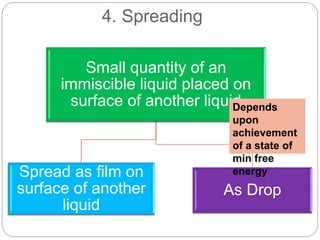
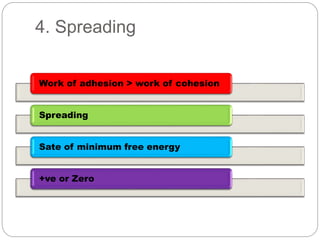
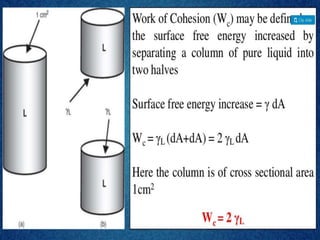
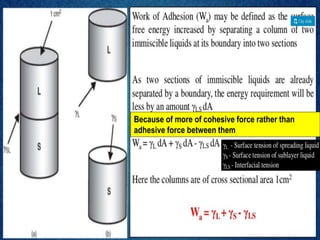
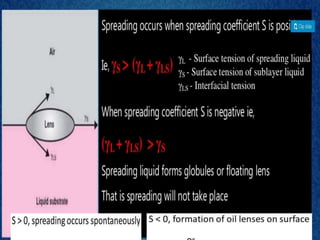
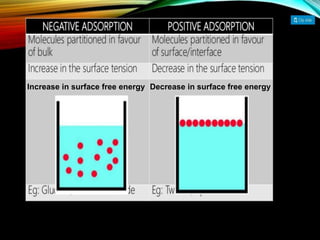
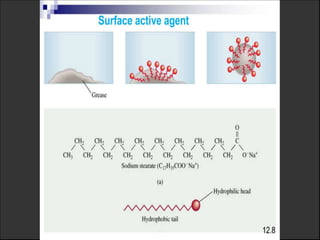
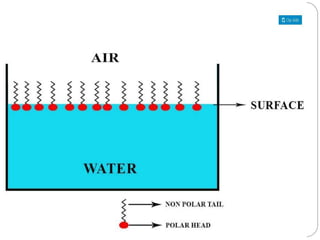
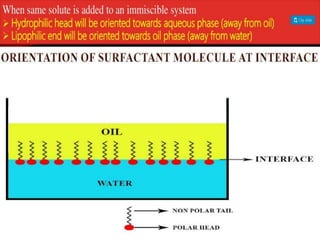
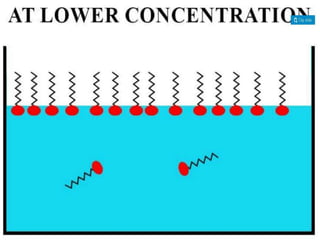
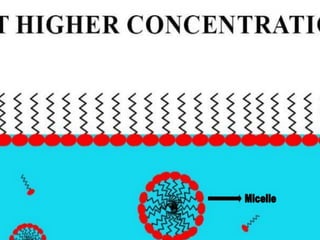
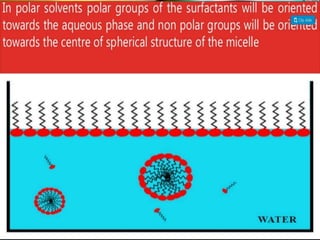
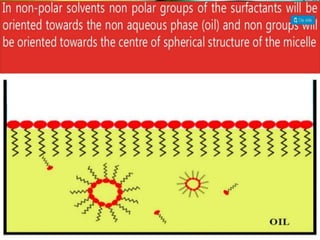
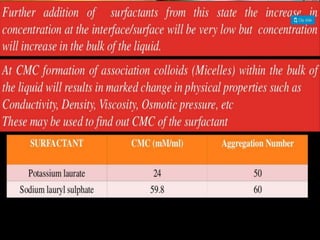
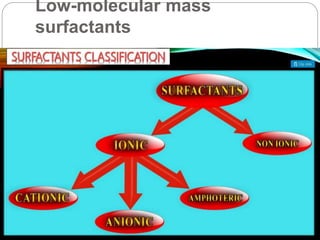
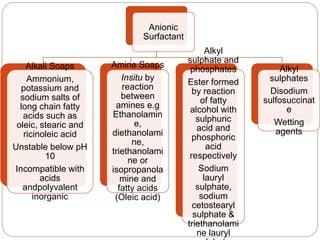
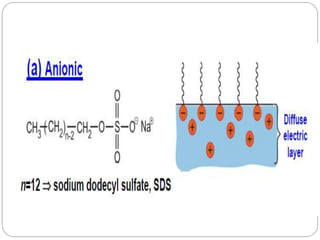
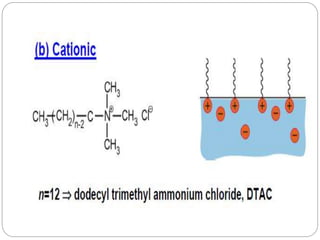
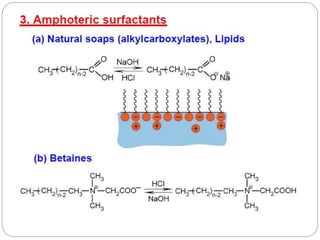
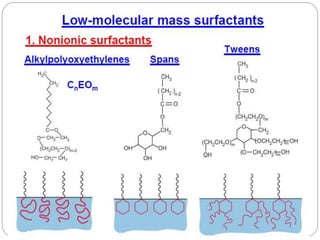
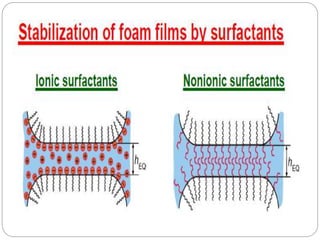
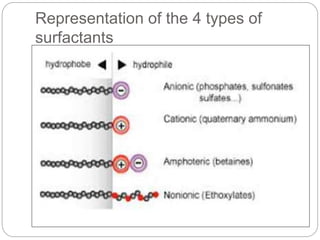
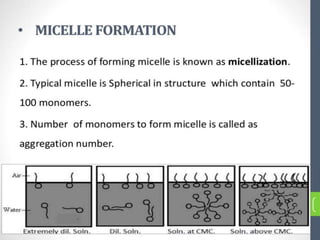
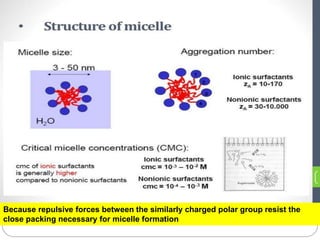
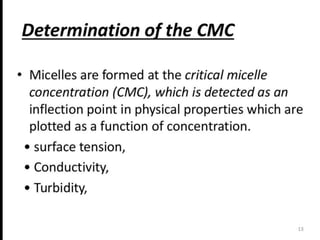
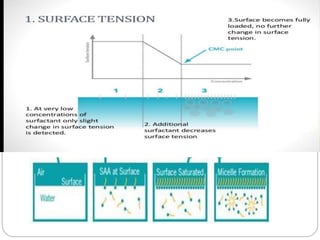
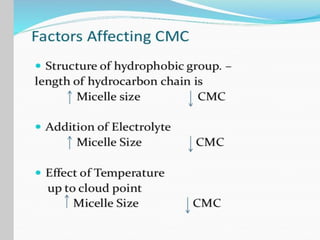
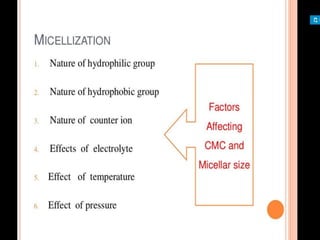
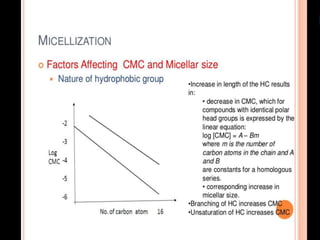
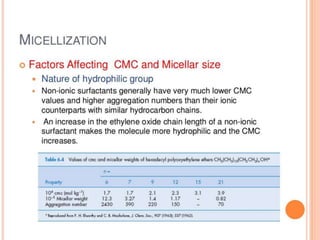
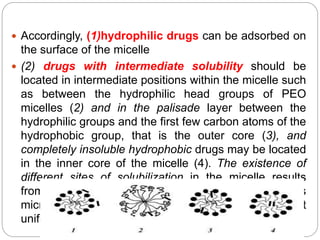
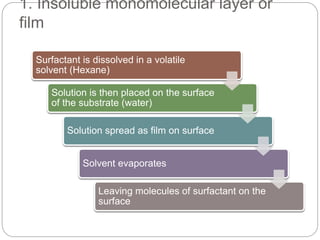
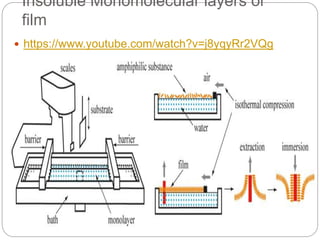
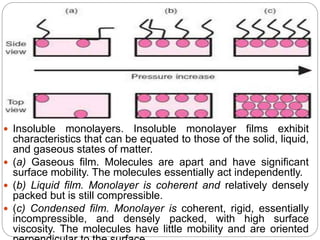
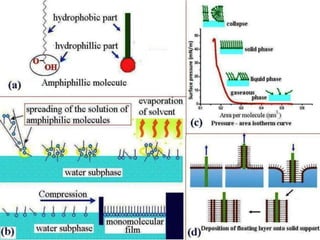
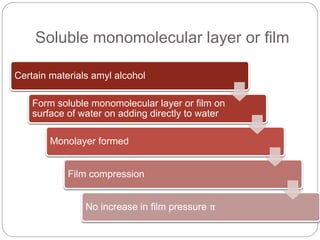
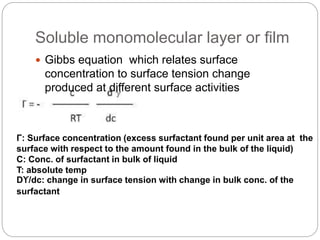
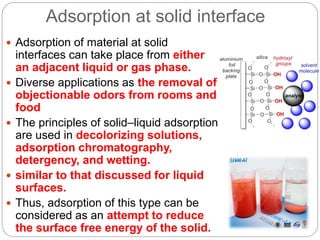
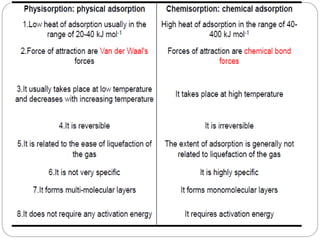
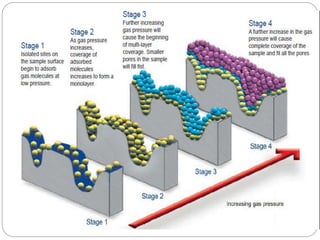
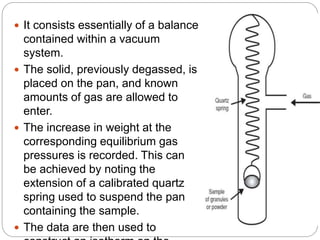
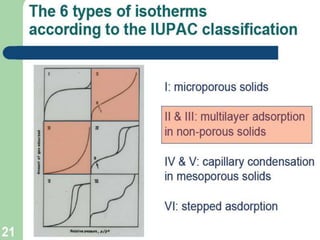
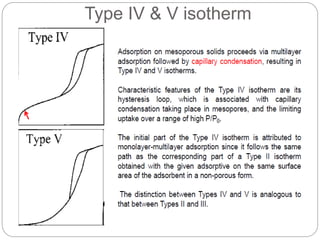
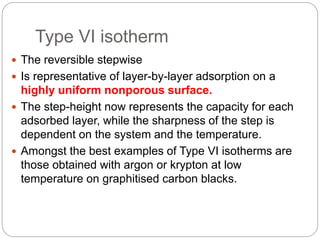
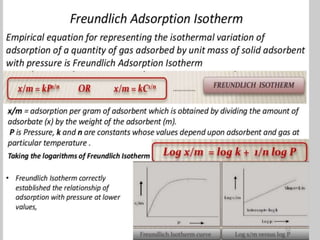
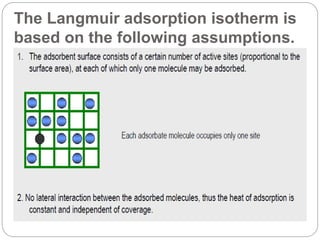
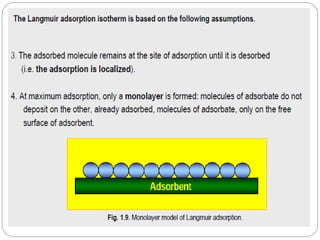
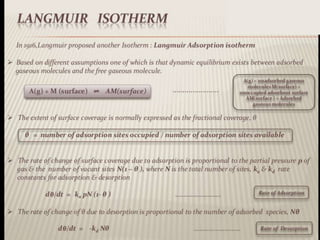
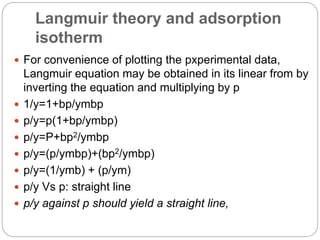
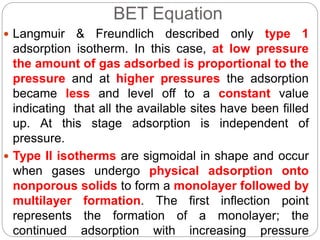
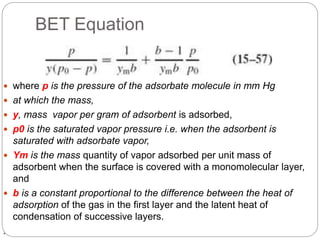
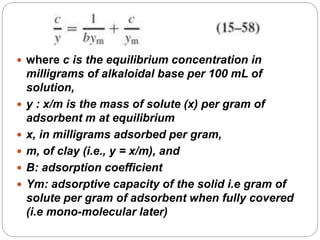
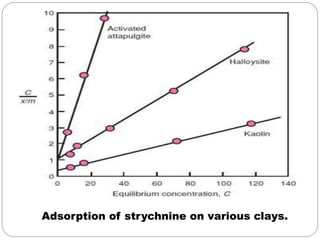
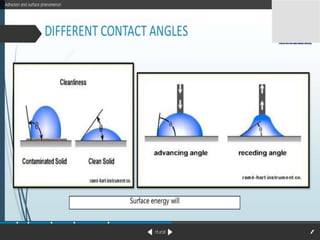
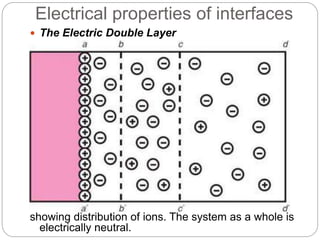
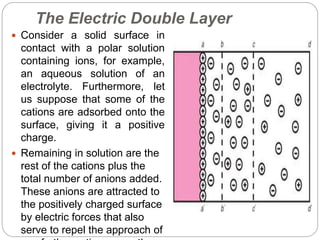
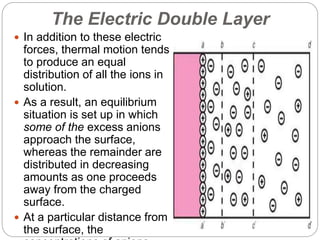
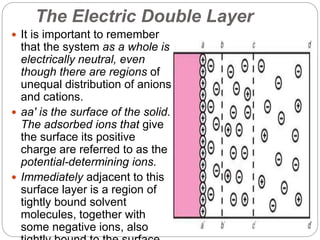
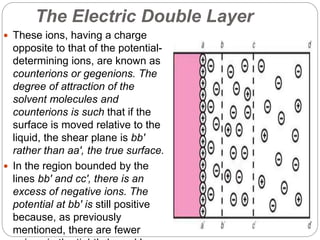
1. An insoluble monomolecular film forms when a slightly soluble material is spread on a liquid surface, such as water. The molecules stand vertically and pack closely, with thickness equaling molecular length.
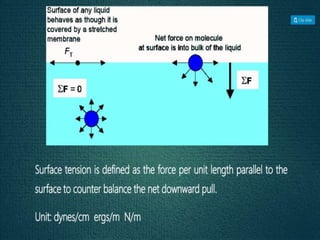
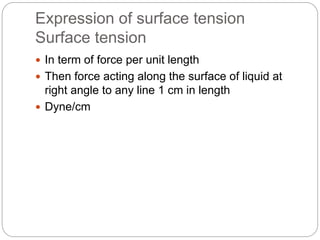
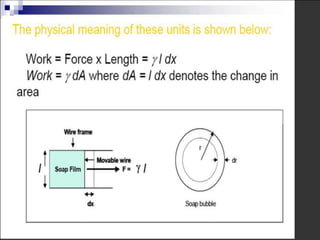
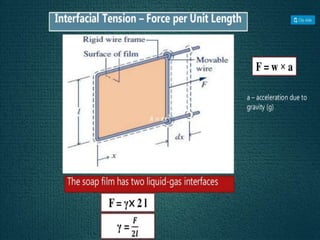
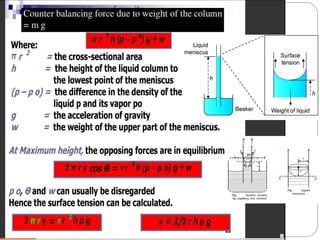
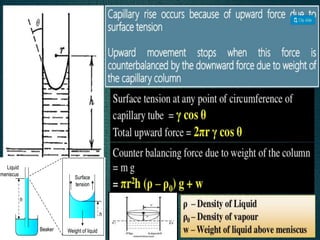
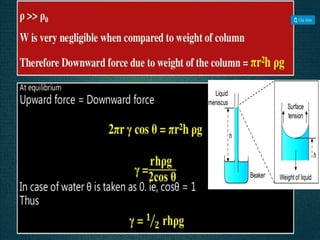
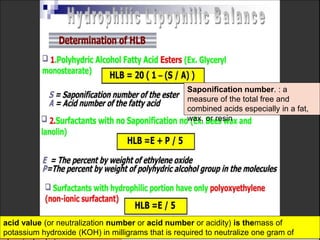
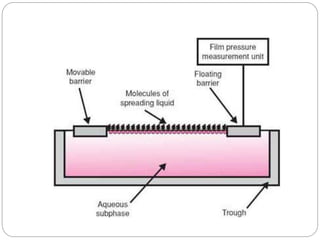
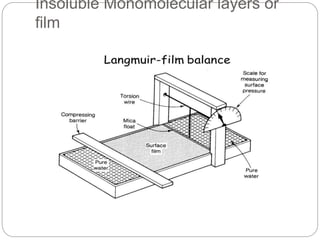
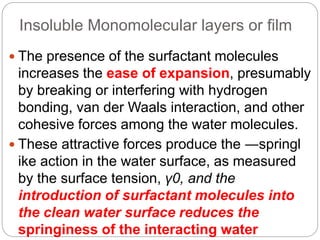
2. Film pressure is measured as the difference between the surface tension of the clean liquid and the surface tension of the liquid covered by the film. The film resists contraction of the clean surface.
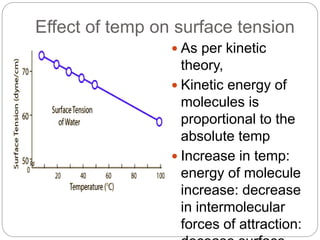
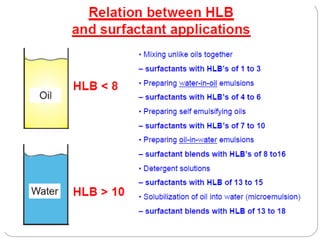
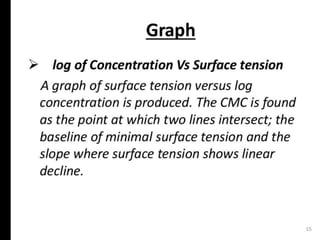
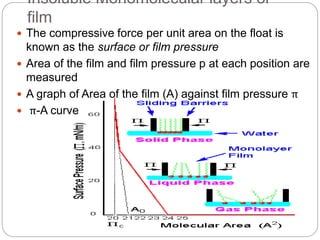
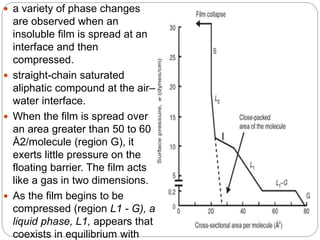
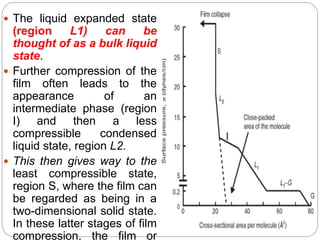
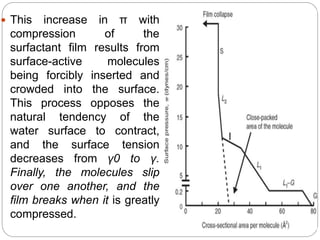
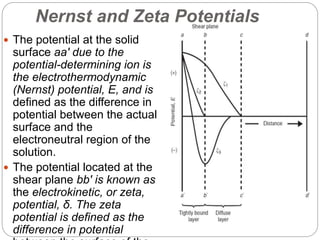
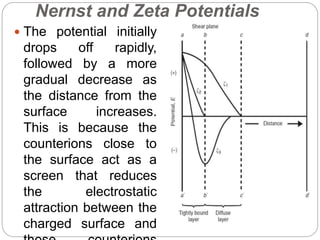
3. A π-A curve plots the relationship between film pressure and film area, showing phase changes as the film is compressed, from a gas-like to liquid-like to solid-like state.