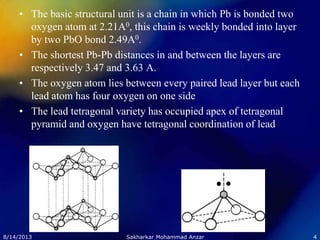
The document discusses the crystal structures of various ceramic compounds. It describes common AB compounds which contain equal numbers of cations and anions. Lead oxide (PbO) exists in two polymorphs, a red tetragonal form and a yellow orthorhombic form, which have different crystal structures. Calcium carbide (CaC2) has the sodium chloride structure with calcium and carbide ions in place of sodium and chloride. Chromium(III) oxide (Cr2O3) adopts the corundum structure consisting of a hexagonal close-packed array of oxide ions and two-thirds of the octahedral sites occupied by chromium cations.