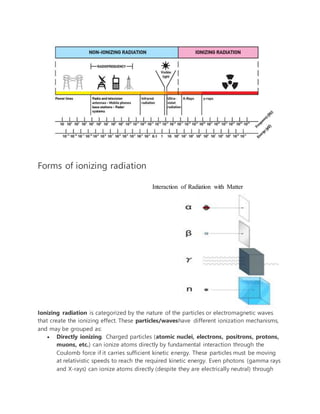
Atomic and nuclear physics are related but distinct fields that describe the structure and behavior of atoms and their nuclei. Atomic physics deals with atoms as systems of electrons and an atomic nucleus, while nuclear physics focuses on the nucleus as a system of nucleons (protons and neutrons). A knowledge of these fields is important for nuclear engineers working with nuclear reactors. The document then provides details on the key topics in atomic and nuclear physics, including fundamental particles, atomic and nuclear structure, mass and energy, radiation, nuclear stability, radioactive decay, and nuclear reactions.