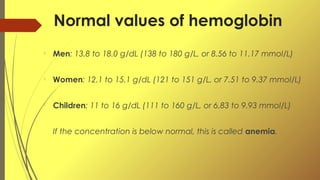
Hemoglobin is a metalloprotein found in red blood cells that transports oxygen throughout the body. It is composed of four subunits, including two alpha and two beta chains, as well as a heme group containing iron. Hemoglobin binds oxygen in the lungs and releases it throughout the tissues to support cellular respiration. Normal hemoglobin levels are 13.8-18.0 g/dL for men and 12.1-15.1 g/dL for women.