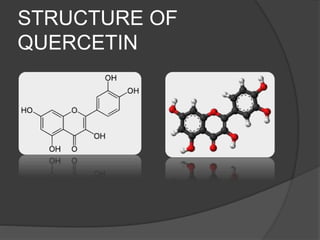
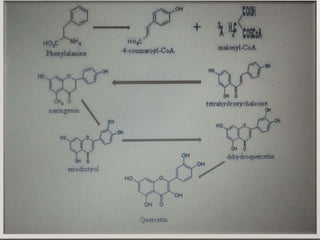
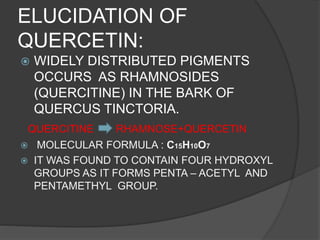
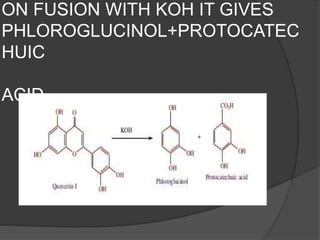
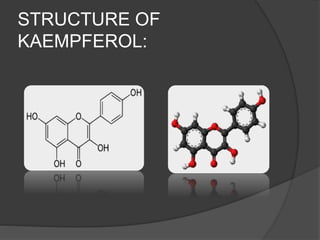
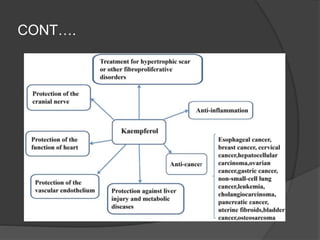
This document discusses quercetin and kaempferol, which are plant flavonoids. It describes their structures, sources, and biosynthesis. Quercetin is found in many fruits and vegetables and has potential health benefits for conditions like asthma, heart disease, and exercise recovery. Kaempferol is present in foods like apples, tea, and broccoli and may help reduce cancer risk. The document also provides details on the biosynthesis pathways that produce these compounds from phenylalanine in plants.