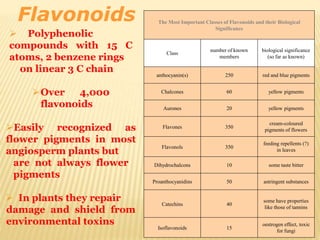
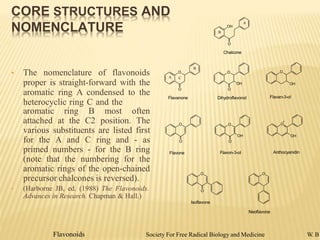
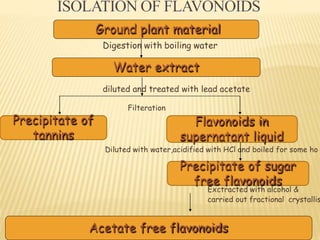
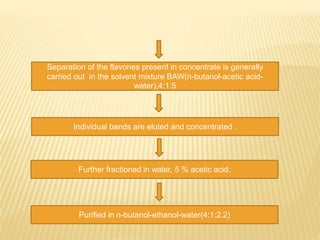
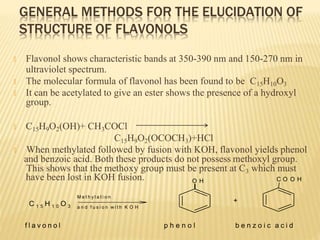
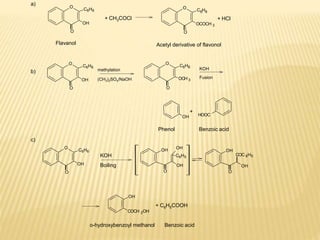
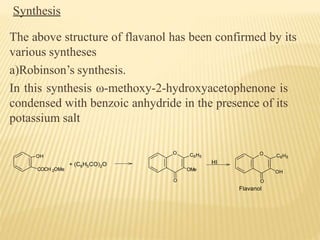
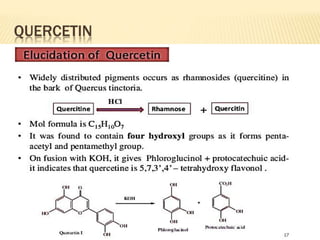
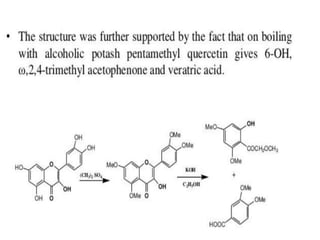
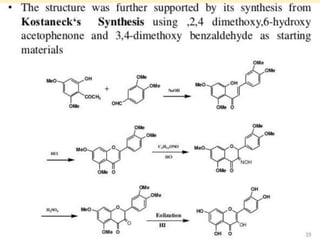
Flavonoids are polyphenolic compounds found in plants that function as pigments. They have 15 carbon atoms arranged in two benzene rings connected by a 3-carbon chain. Flavonoids are common in flowers but also found in other plant parts. They act as antioxidants and have anti-inflammatory, antiviral, and antitumor properties. Common types of flavonoids include flavones, flavonols, flavanones, and anthocyanidins. Quercetin is a flavonol found in many fruits and vegetables that has demonstrated anti-inflammatory, antiviral, anti-cancer, and anti-allergy effects in preliminary research.