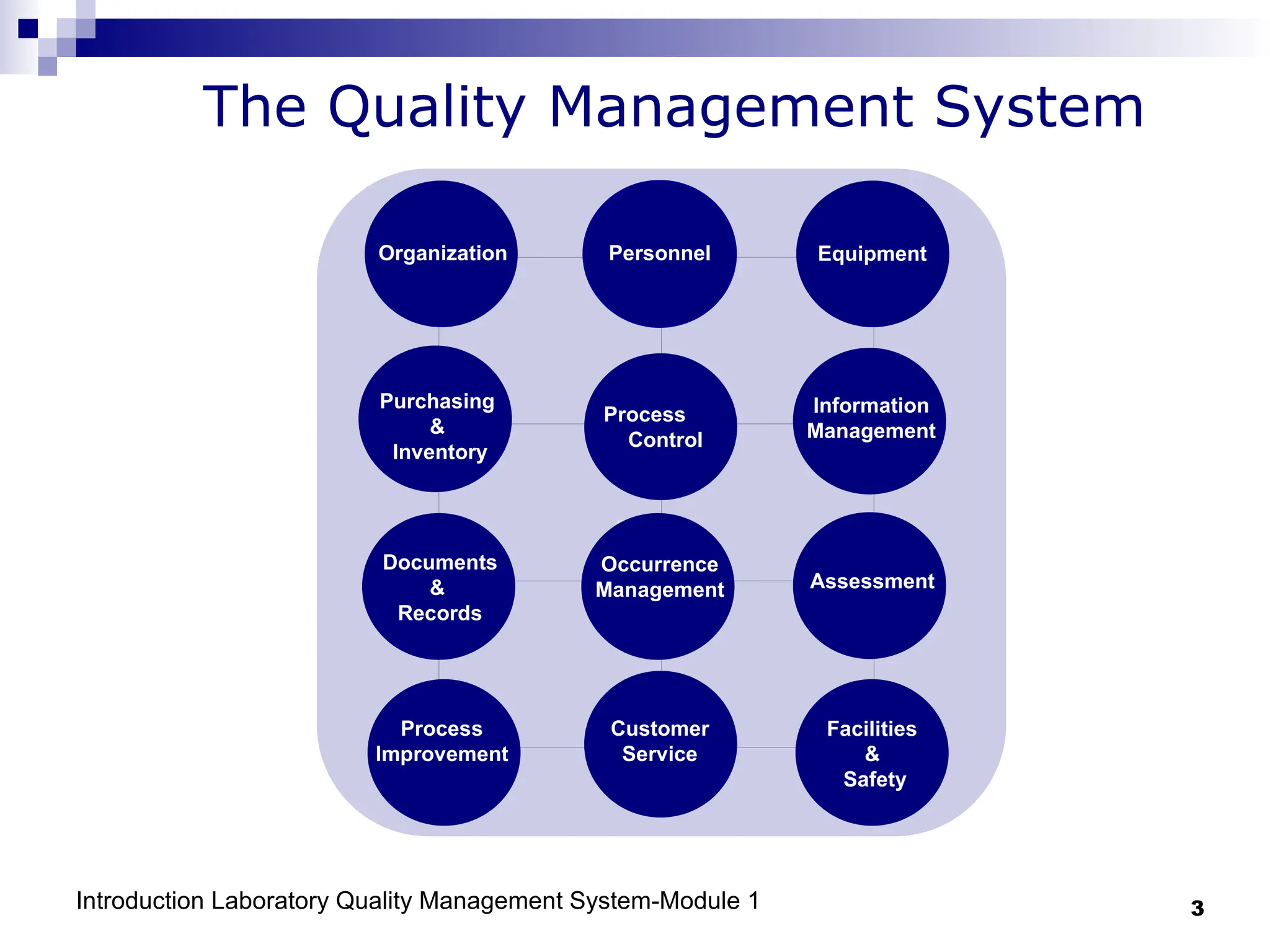
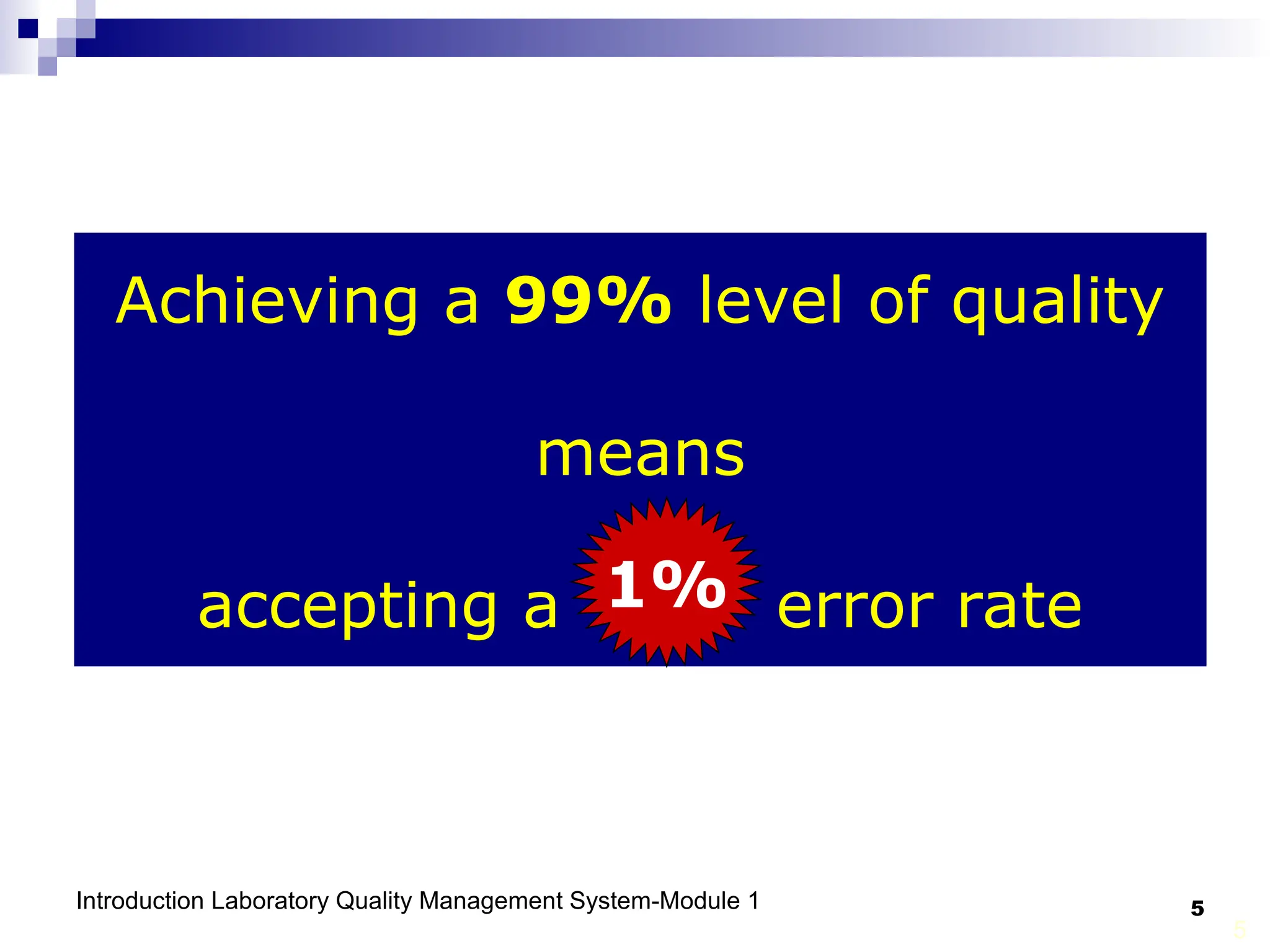
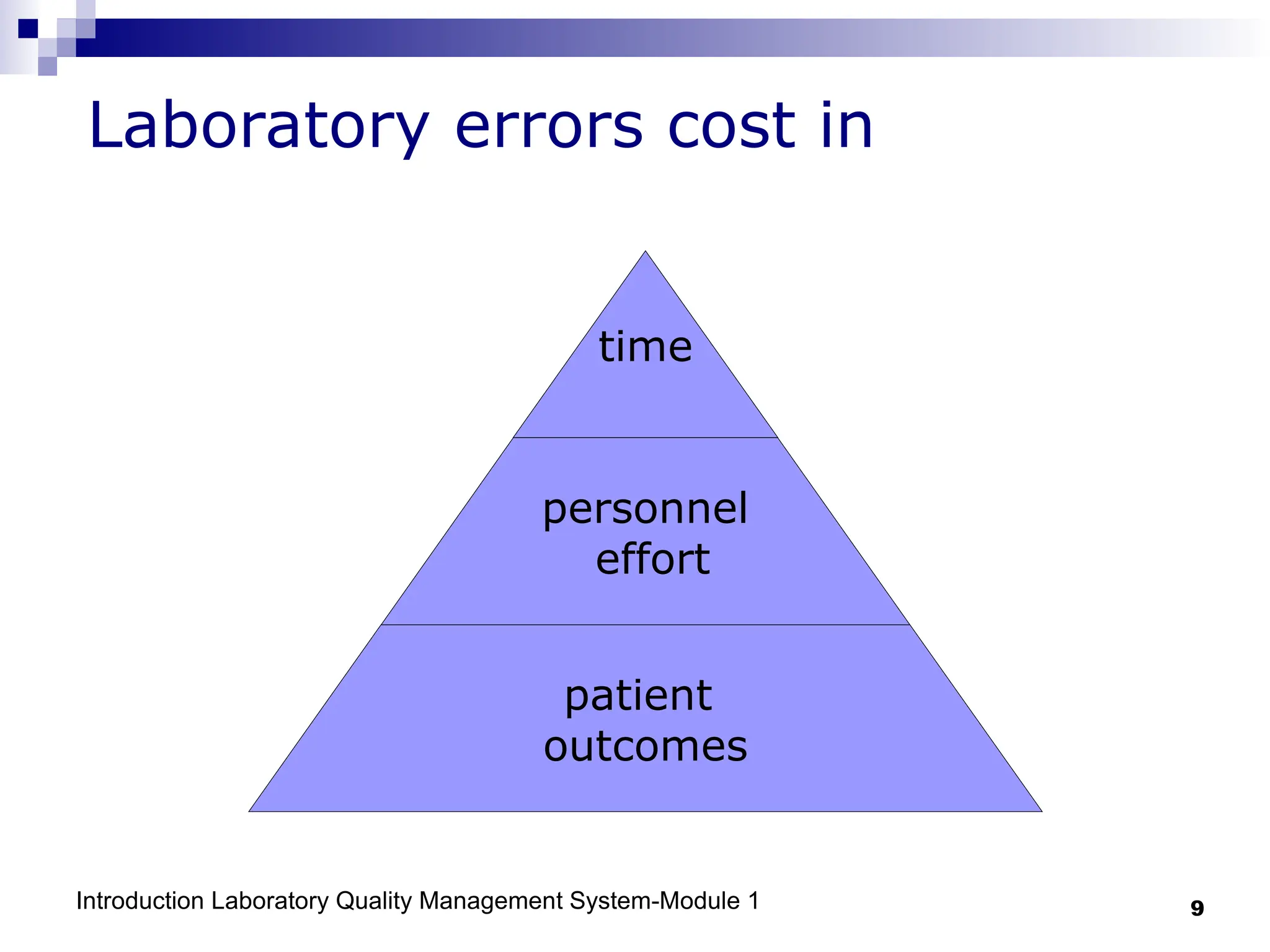
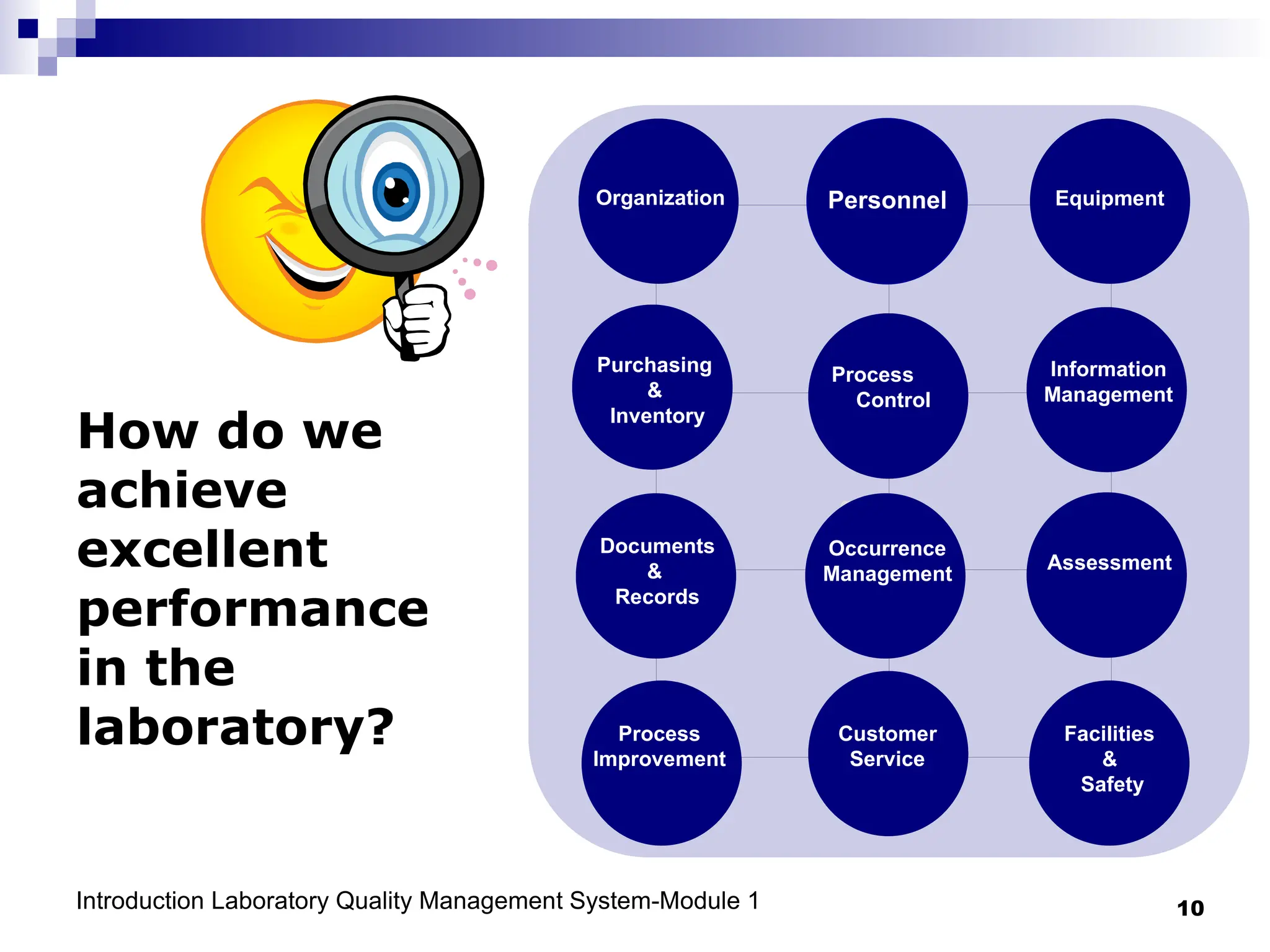
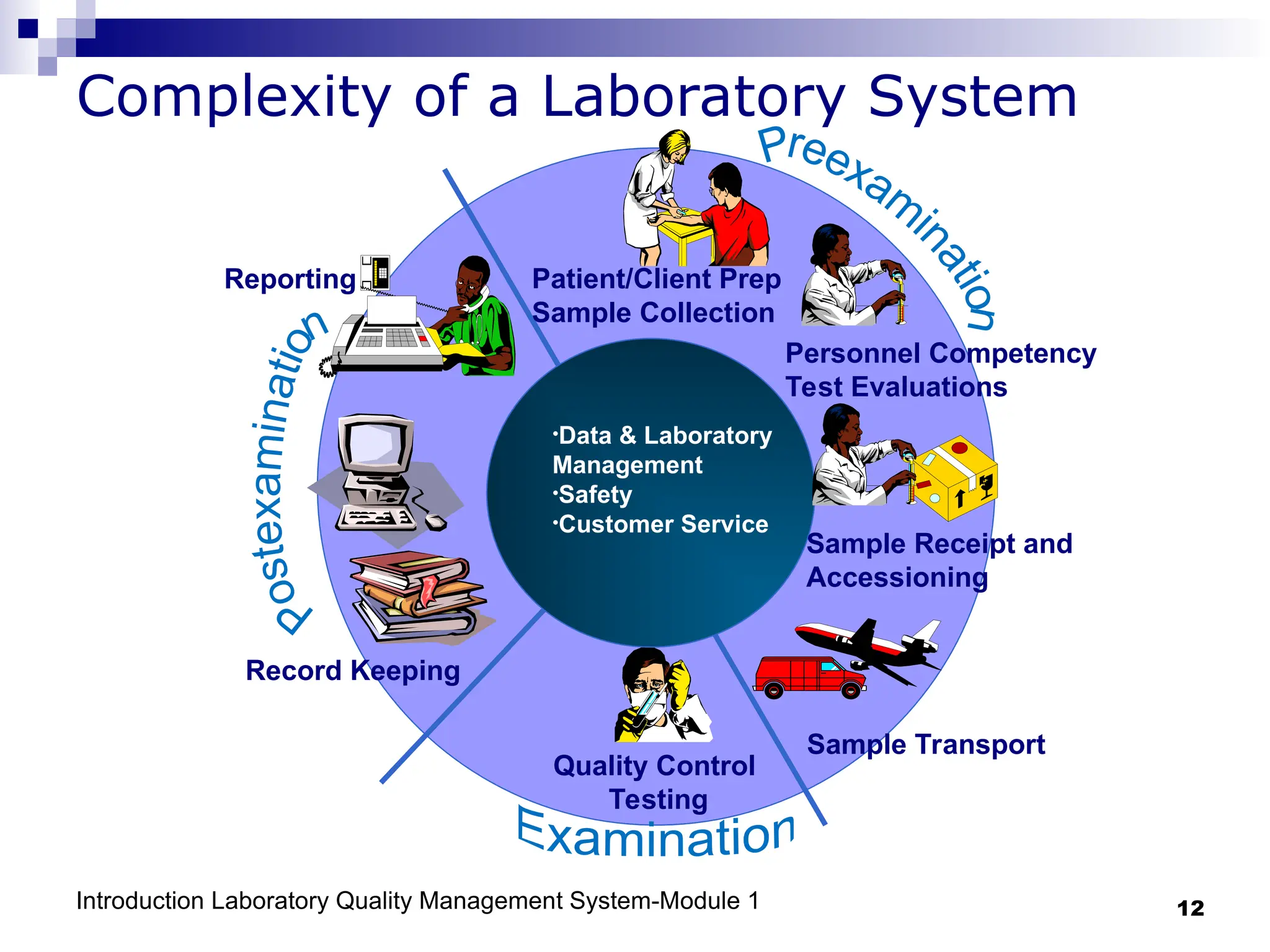
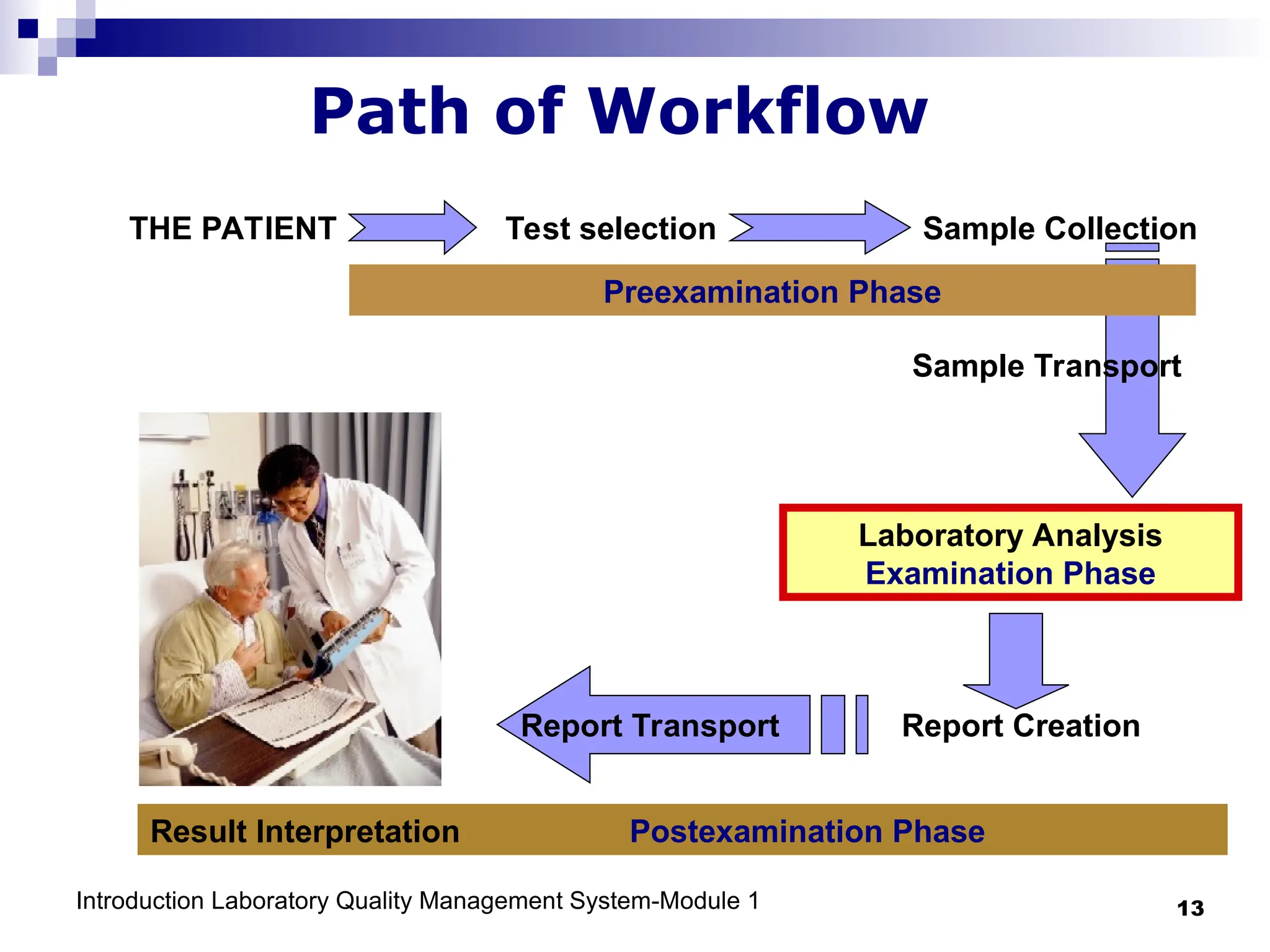
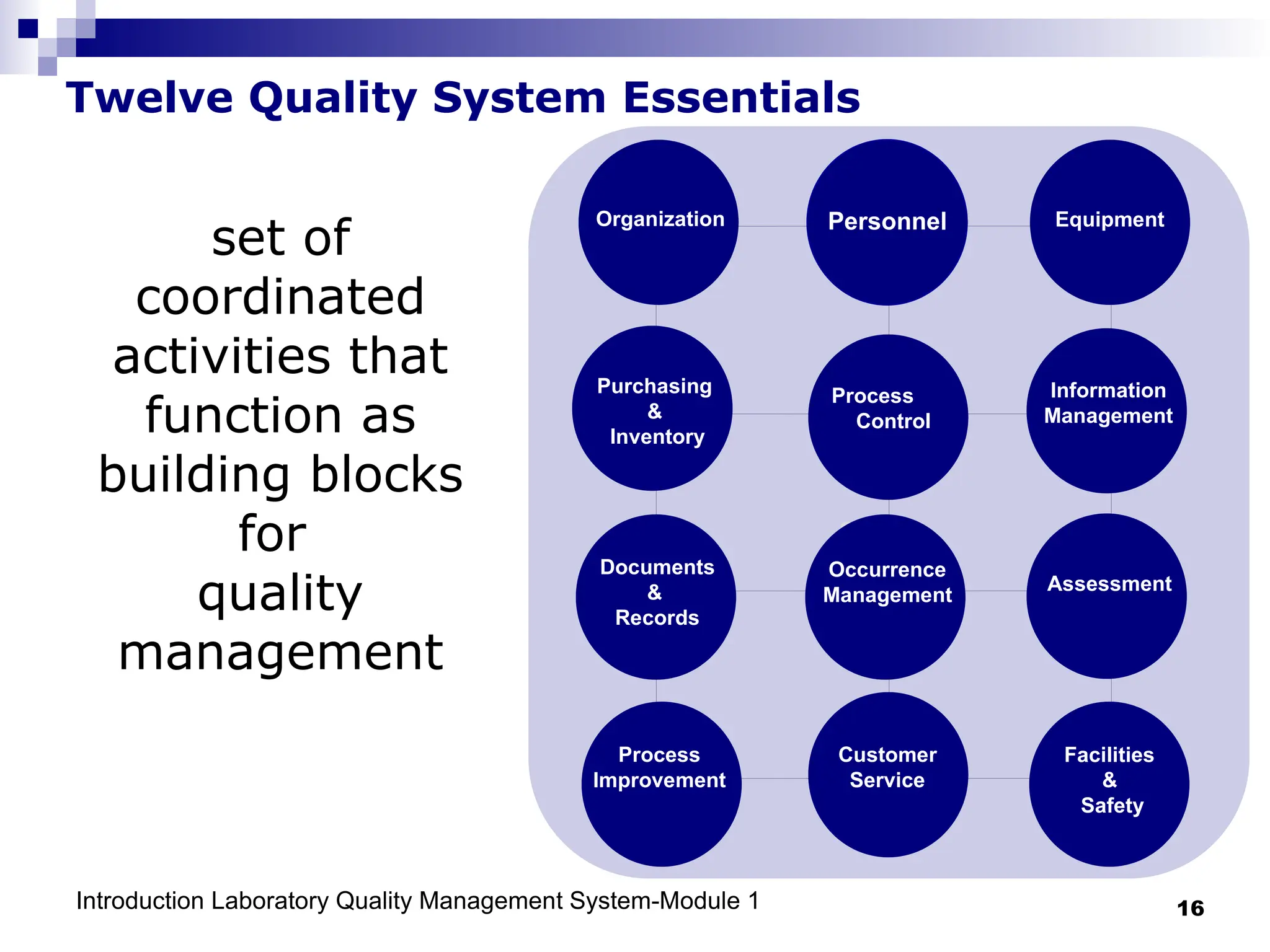
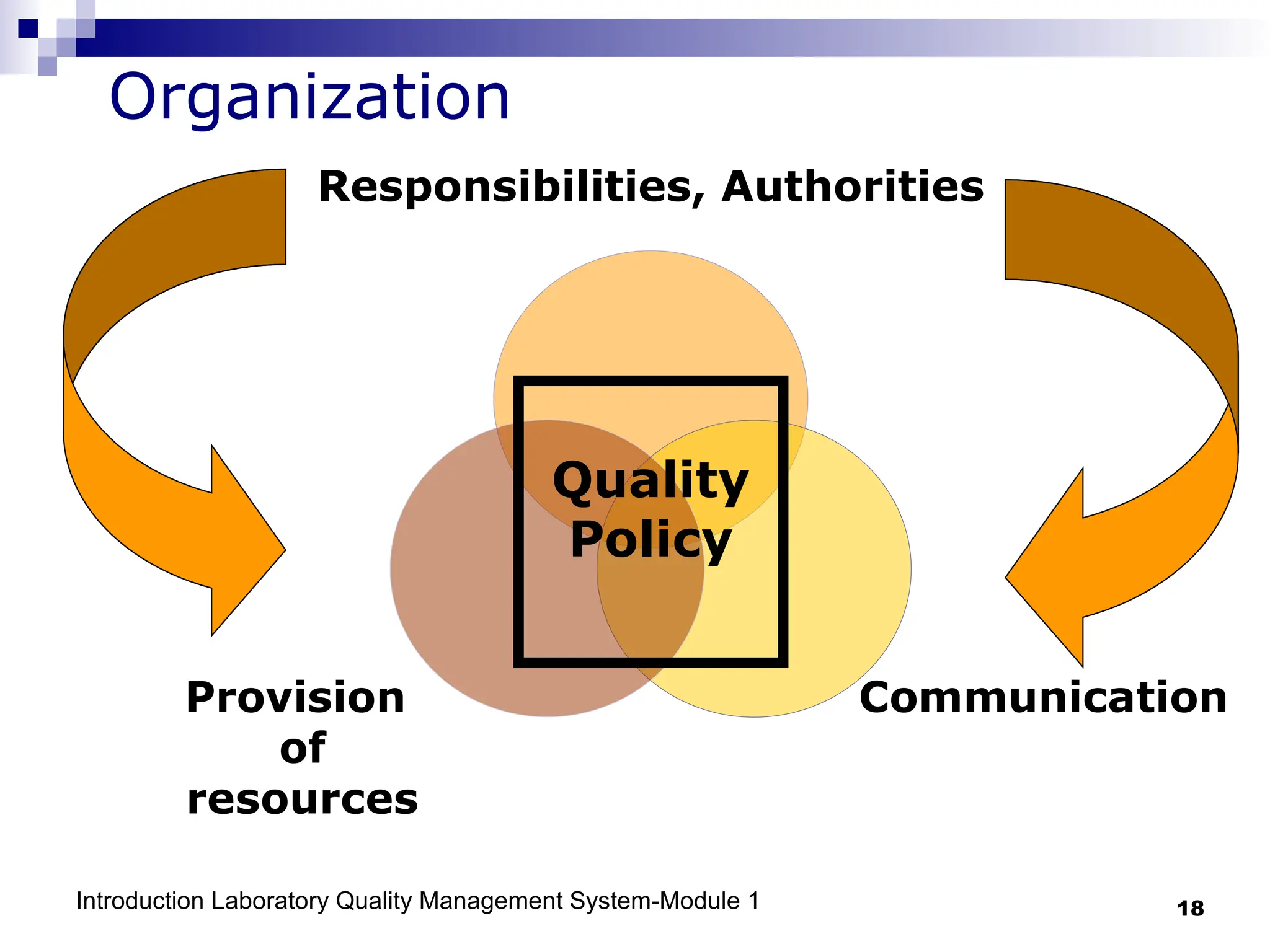
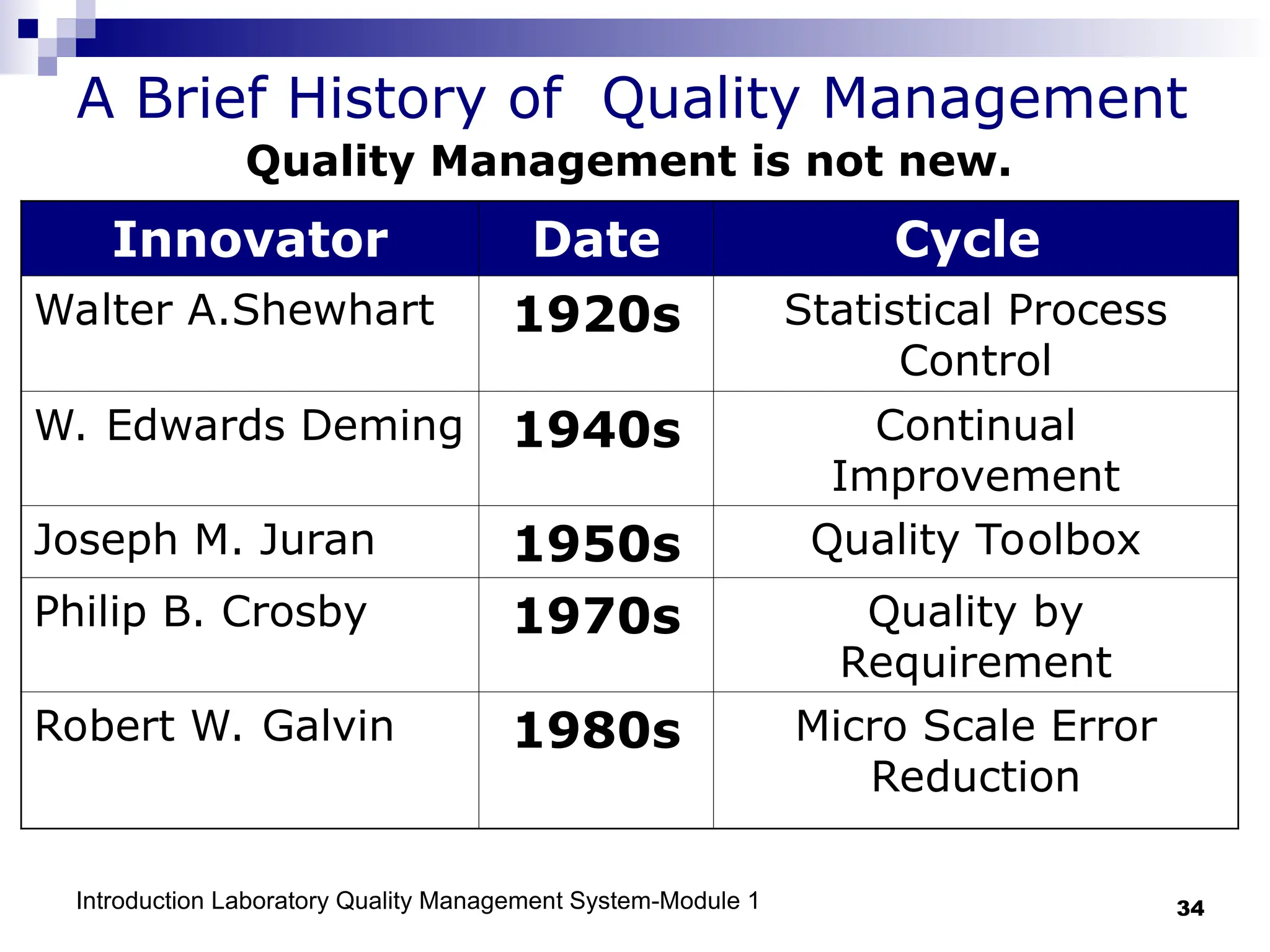
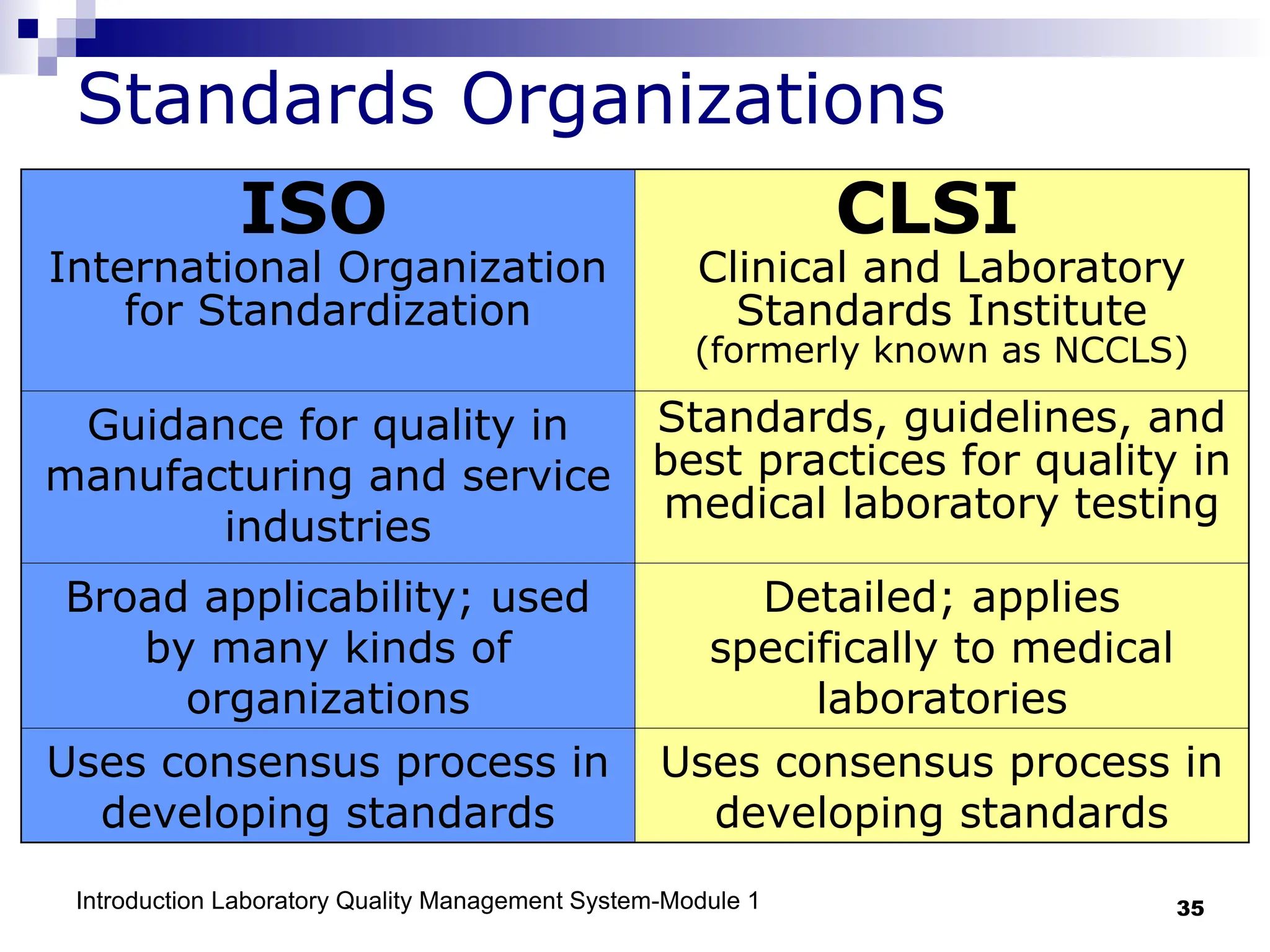
The document outlines the key components and importance of implementing a Laboratory Quality Management System (LQMS), focusing on achieving accurate, reliable, and timely laboratory results. It discusses the essential elements of the quality system, including organization, personnel, equipment, process control, and continual improvement, as well as their relationship to ISO and CLSI standards. The text emphasizes that while quality management does not guarantee an error-free laboratory, it helps detect and prevent errors from recurring.