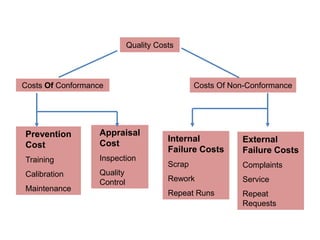
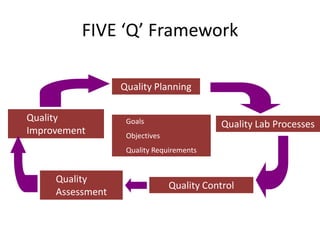
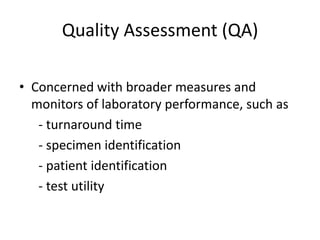
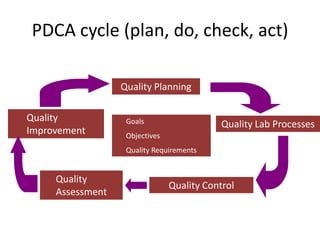
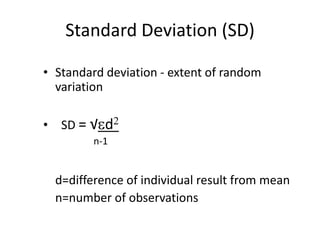
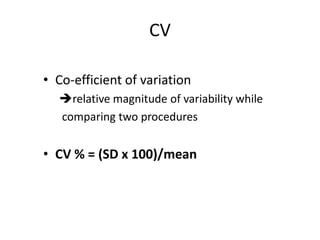
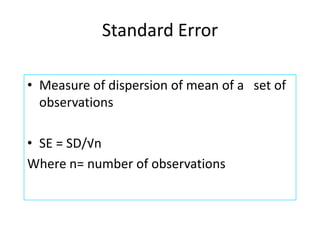
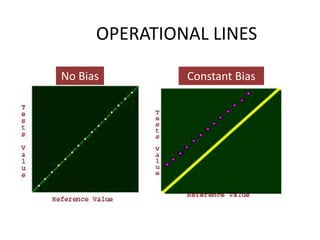
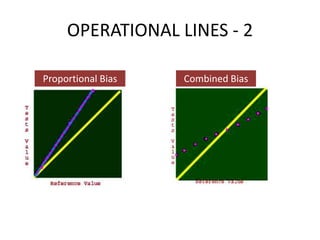
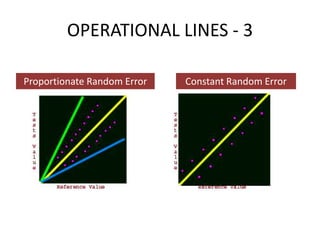
This document discusses quality control in healthcare laboratories. It defines key terms like accuracy, precision, bias, and error. It explains the different types of quality costs like prevention, appraisal, and failure costs. It introduces the concept of total quality management using the five "Q" framework of quality planning, assessment, control, improvement and processes. It emphasizes that 85% of problems are due to process issues that require management changes. Quality control procedures like calibration checks and statistical process control are discussed. The document promotes using the PDCA (plan-do-check-act) cycle for continuous quality improvement.