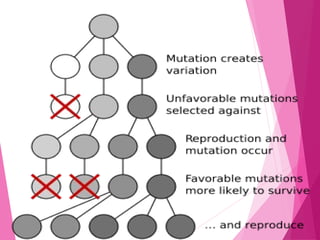
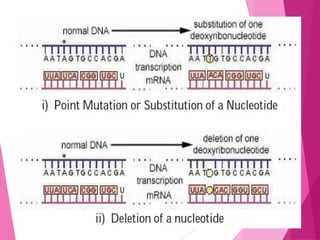
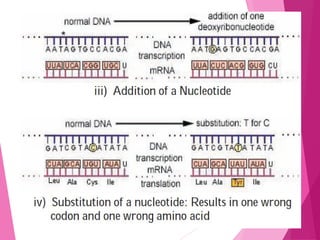
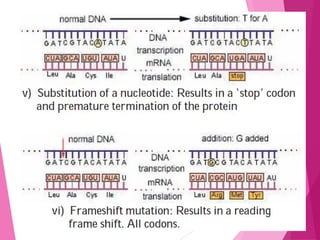
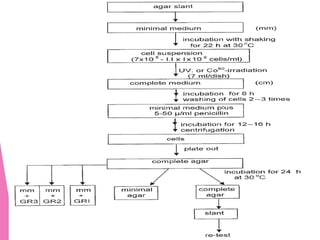
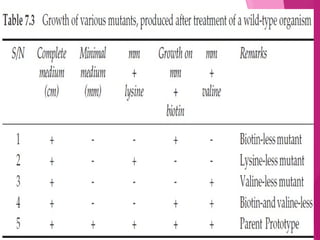
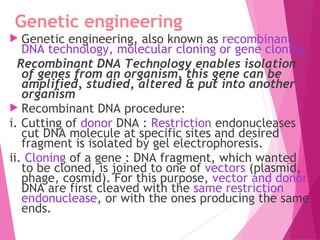
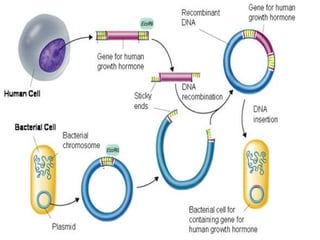
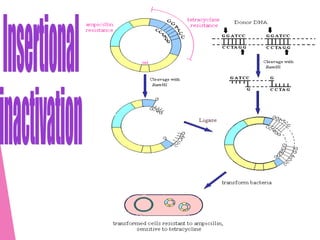
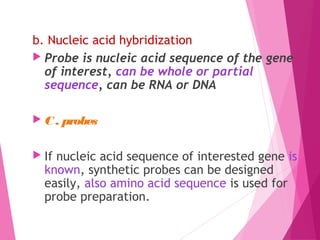
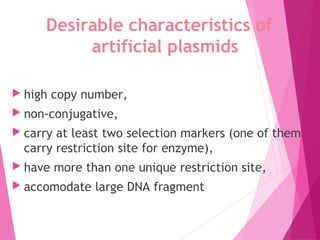
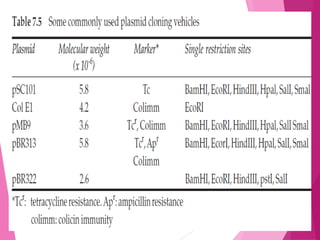
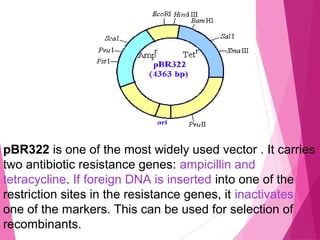
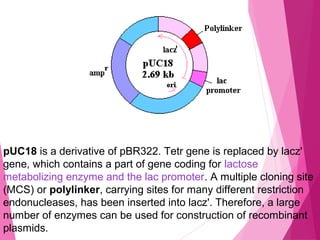
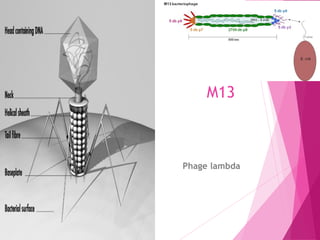
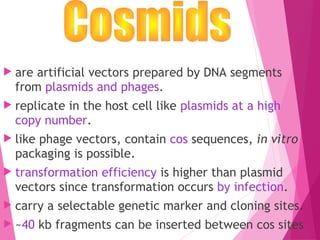
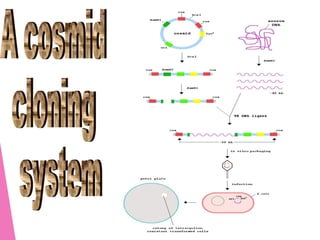
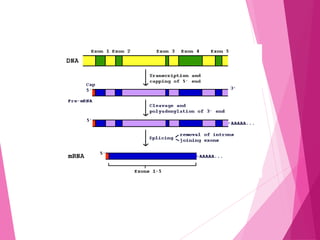
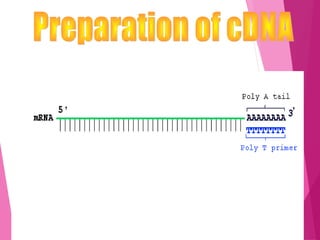
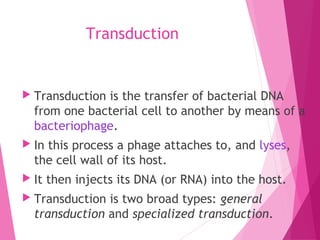
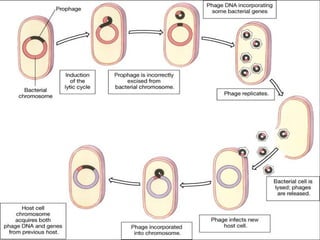
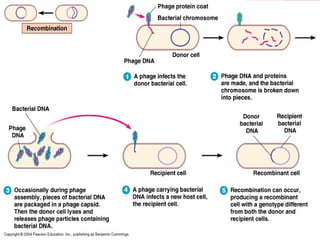
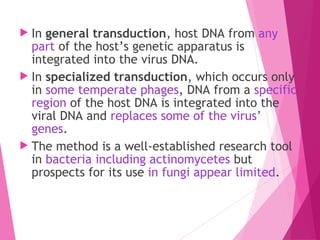
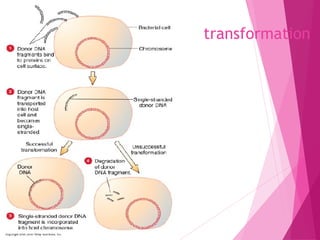
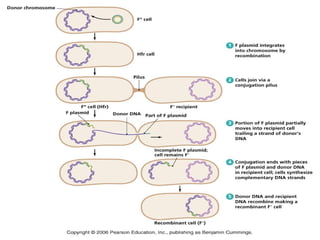
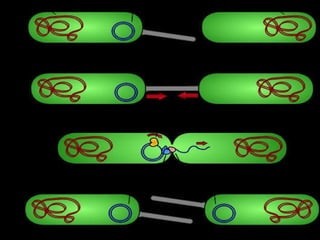
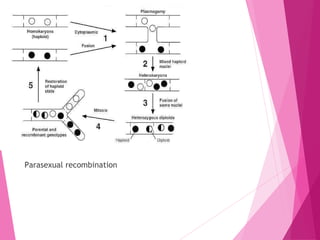
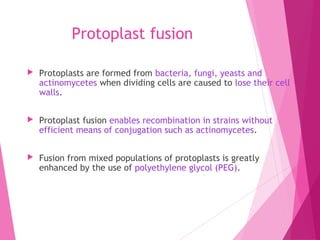
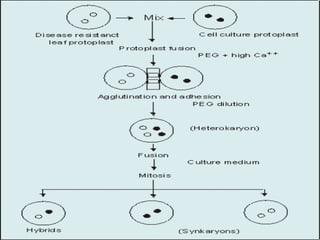
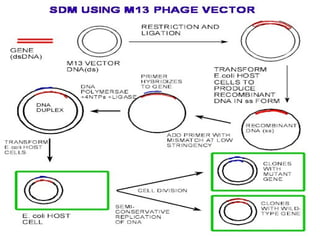
This document discusses various methods for improving microbial strains, including selecting naturally occurring variants, manipulating existing genetics, and introducing new genetics. It focuses on mutation and selection techniques like chemical or UV mutagenesis followed by selection on selective media. Genetic engineering techniques are also summarized, including restriction digestion, ligation into vectors, transformation, and screening of recombinants. Common vectors like pBR322, pUC18, phages like M13, and cosmids are described. The overall goal is to outline strategies for isolating industrially useful microbial mutants.