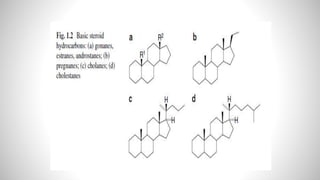
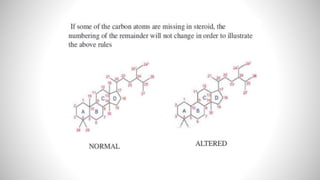
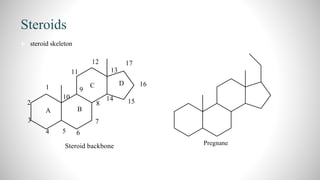
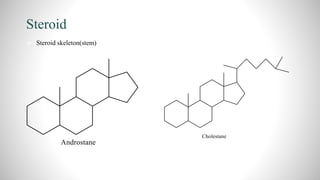
1) Steroids are organic compounds that contain a cyclopentanoperhydrophenanthrene nucleus. They include hormones like vitamin D, bile acids, and sex hormones.
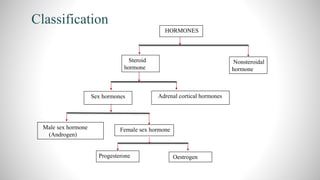
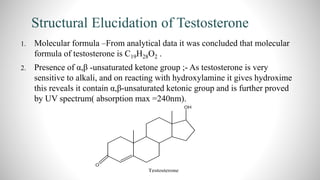
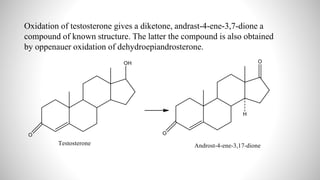
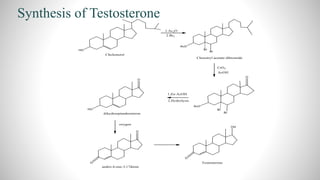
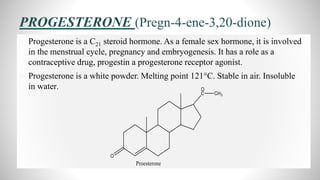
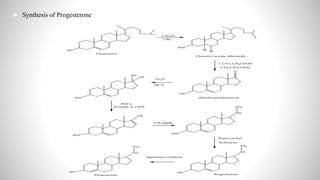
2) The document discusses the structural elucidation of the steroid hormones testosterone and progesterone. Testosterone is the main male sex hormone, while progesterone is a female sex hormone involved in the menstrual cycle and pregnancy.
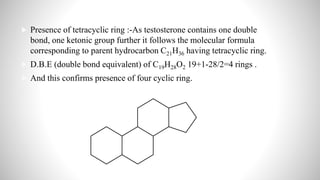
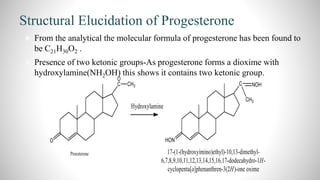
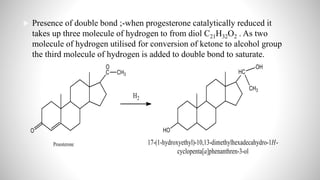
3) Through analysis and reactions, the key features of each steroid's structure were determined, including functional groups, double bonds, and the characteristic tetracyclic steroid nucleus.