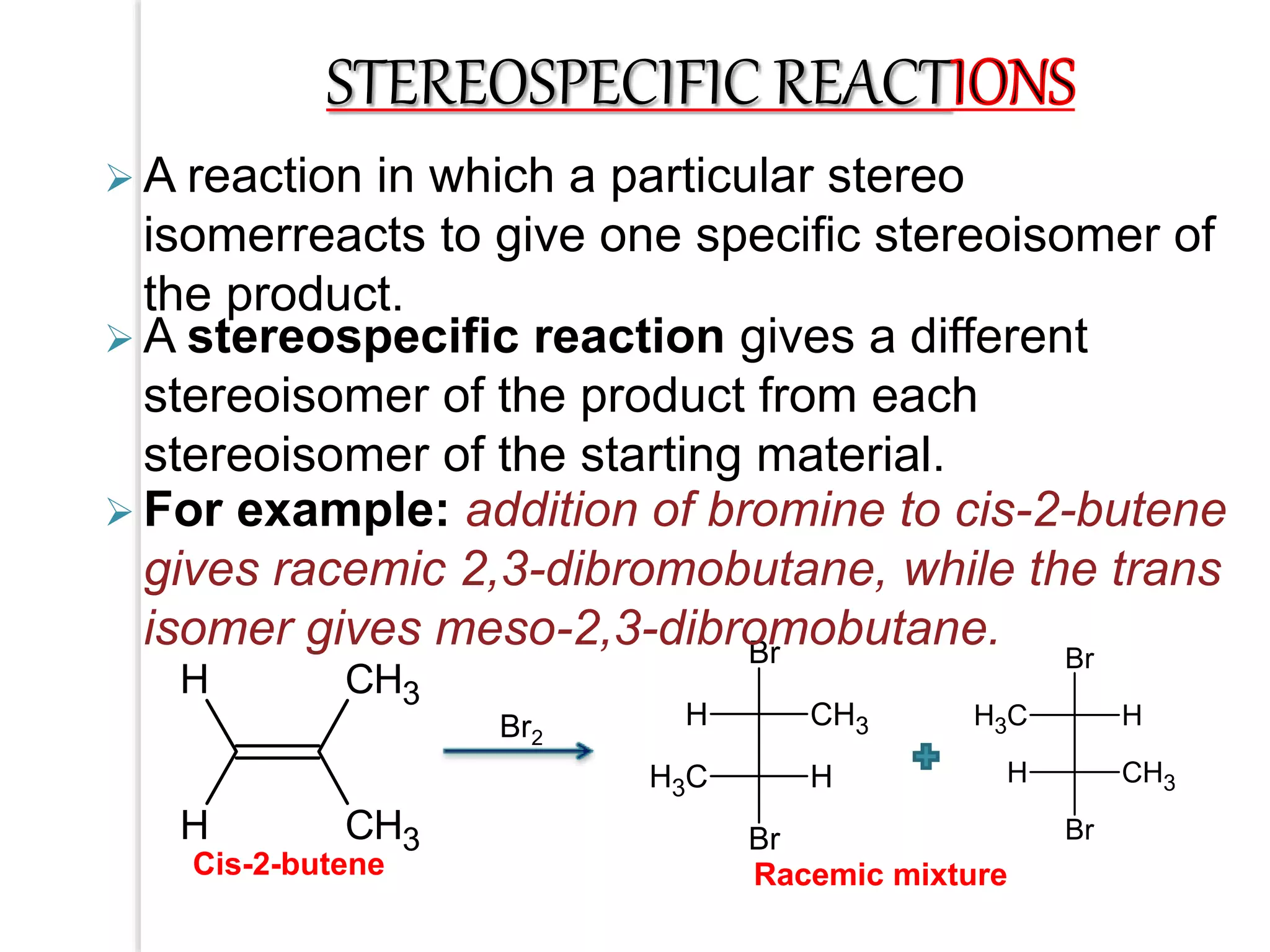
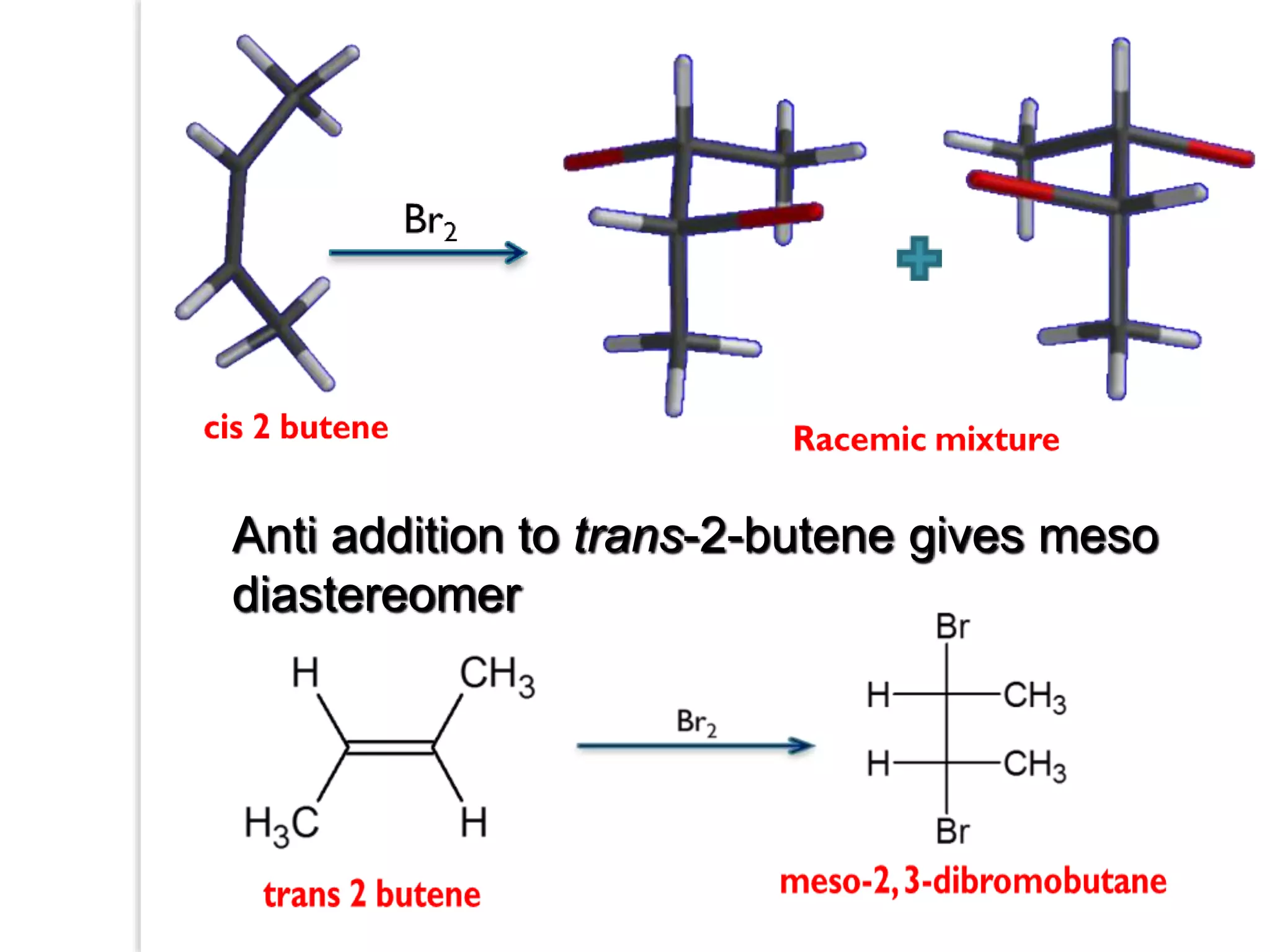
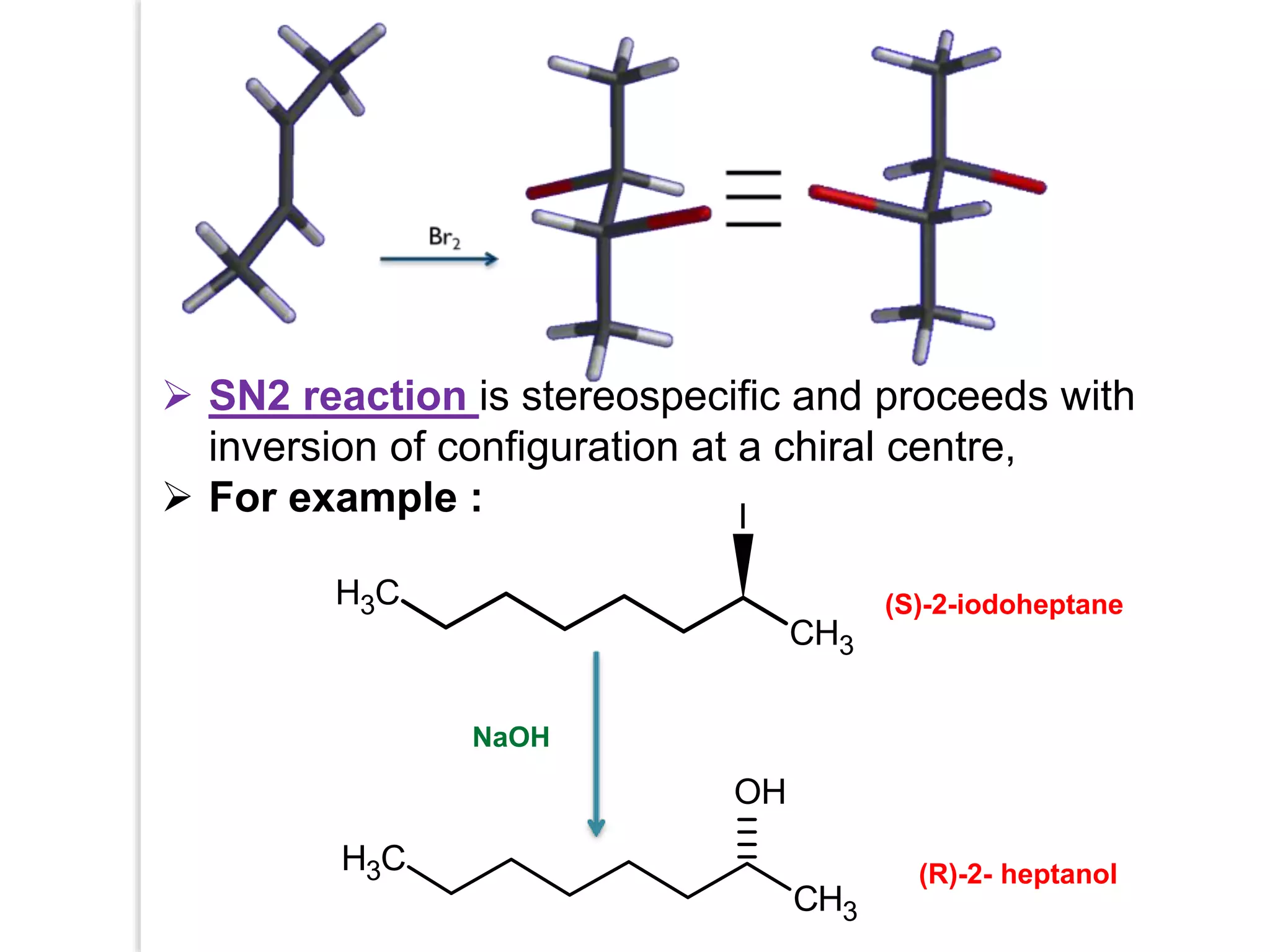
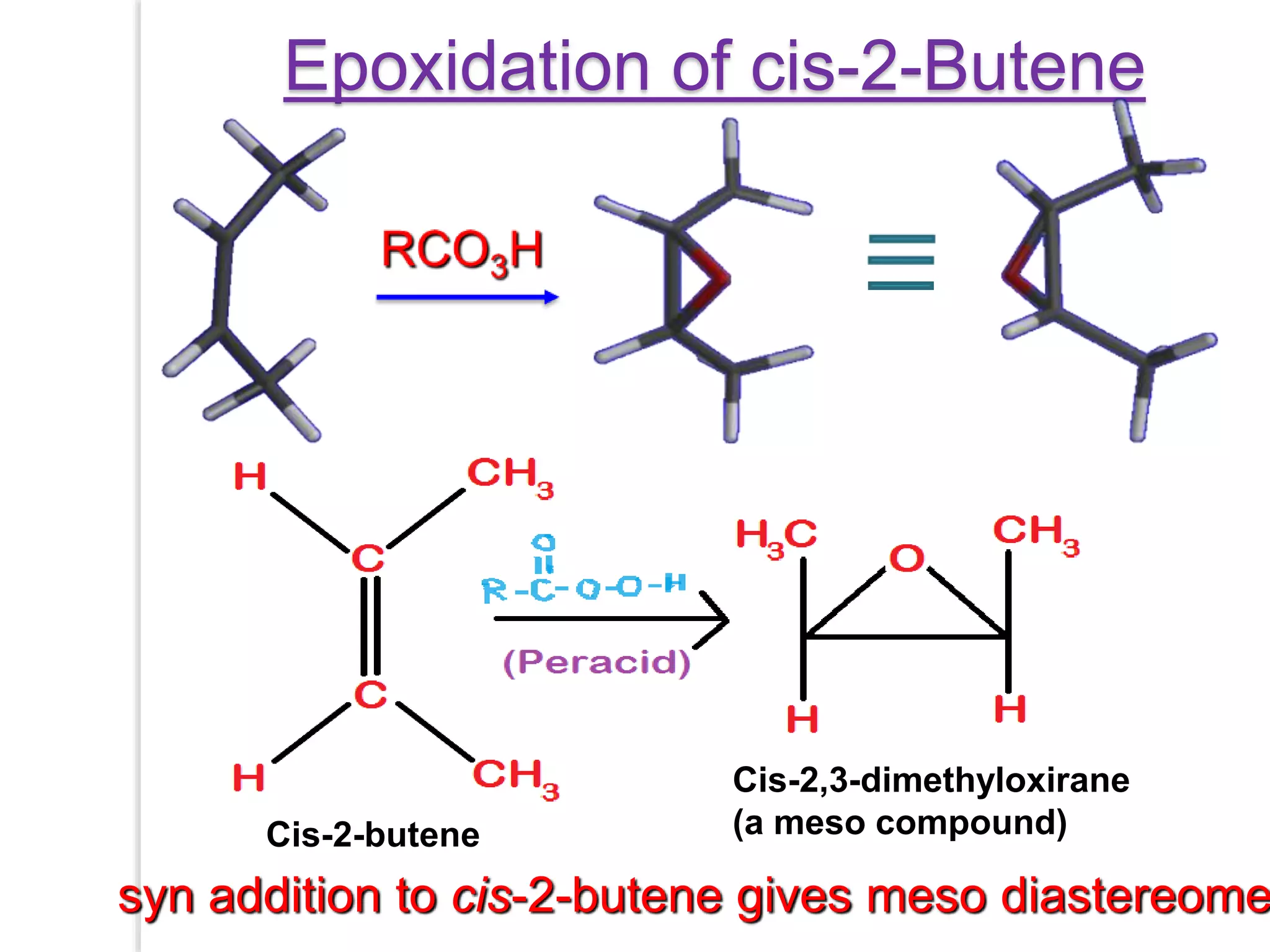
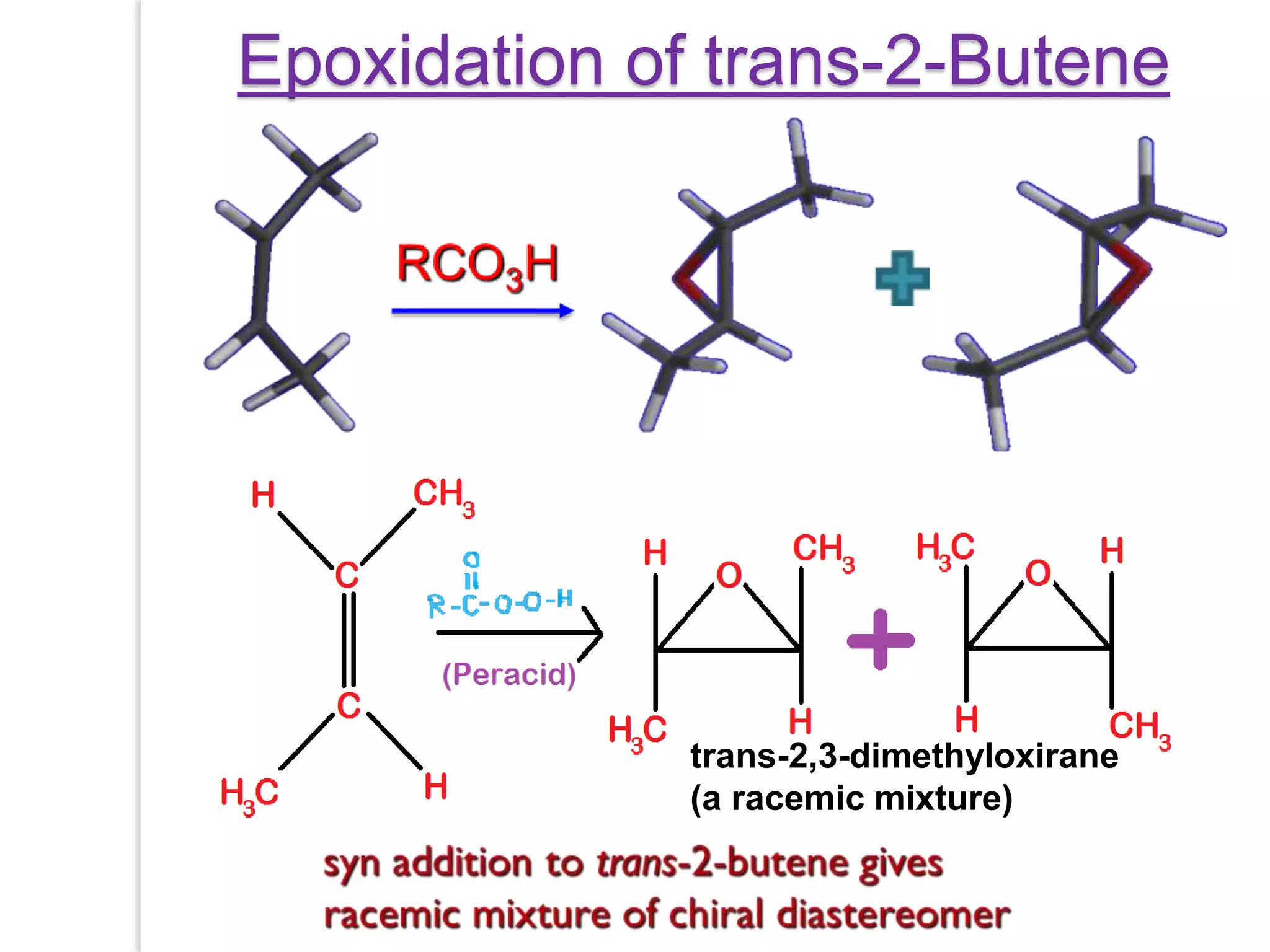
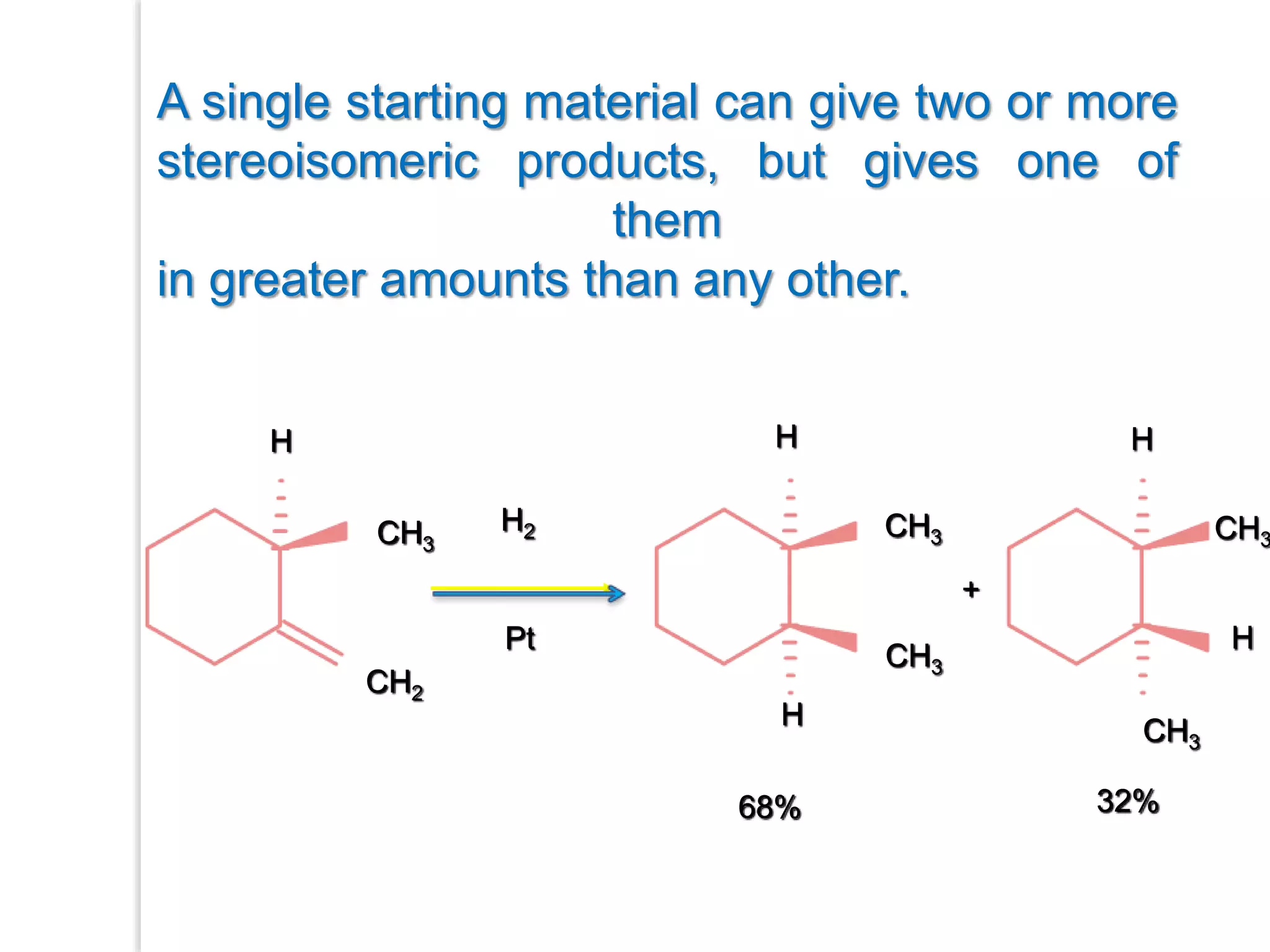
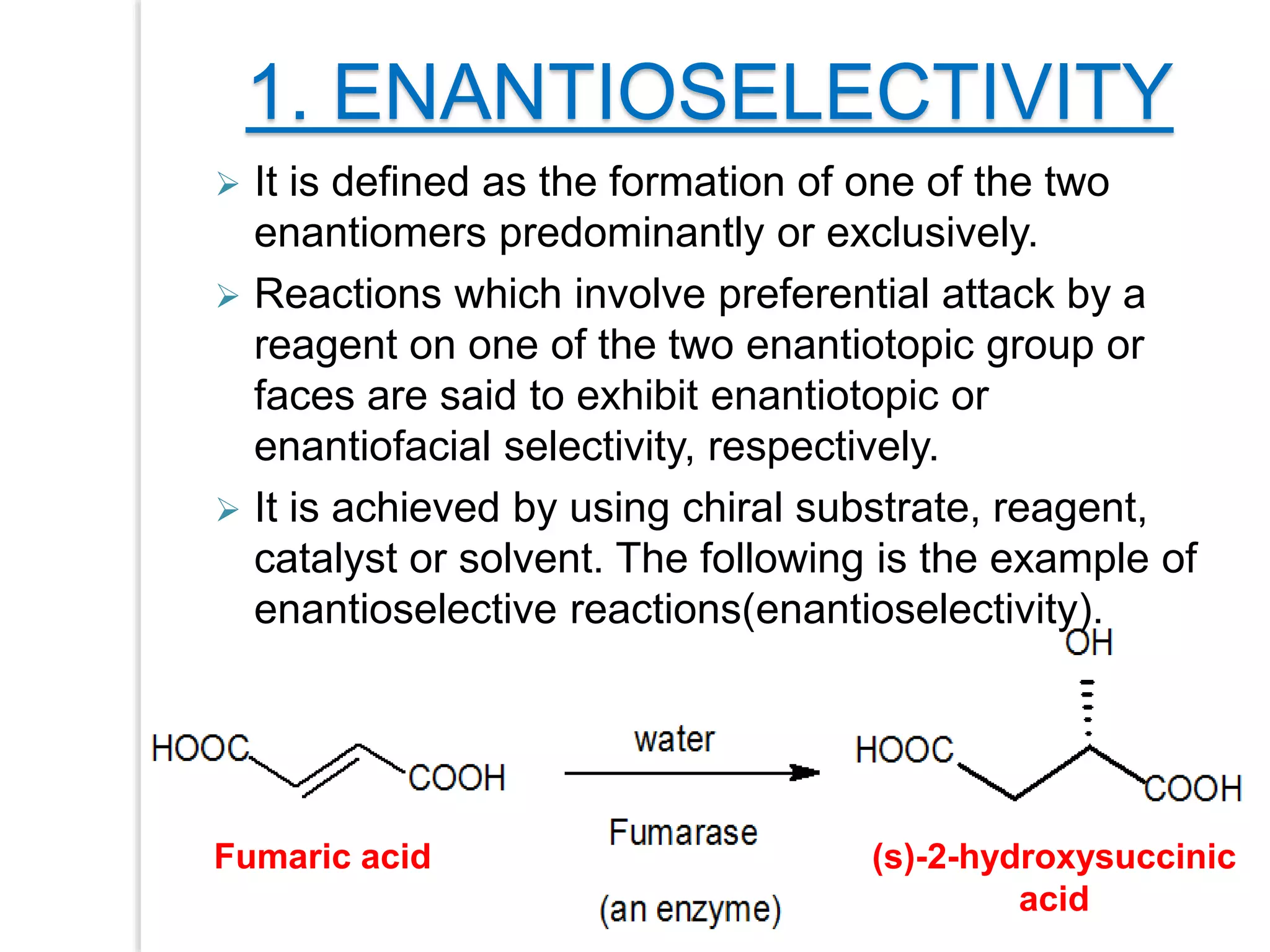
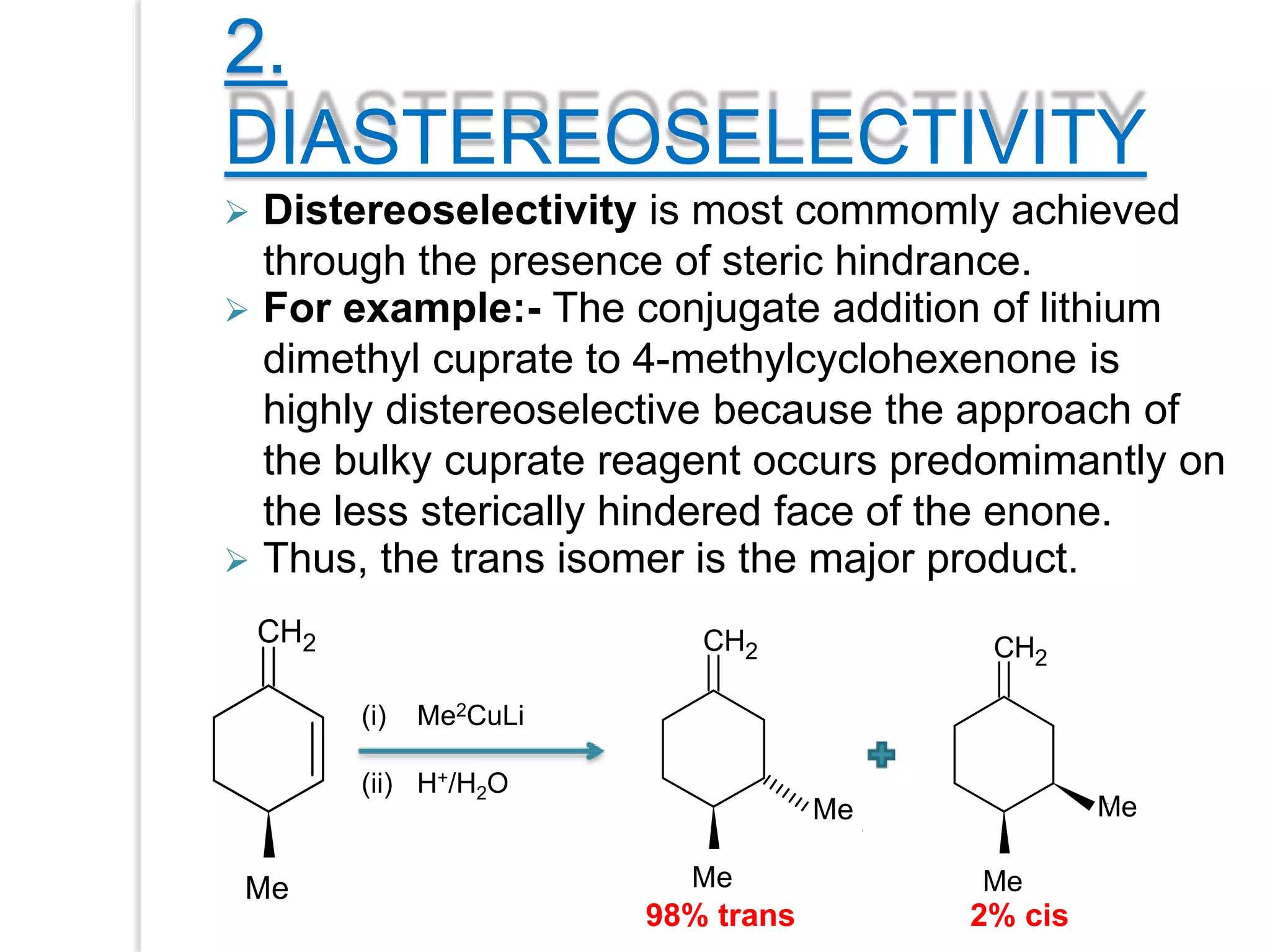
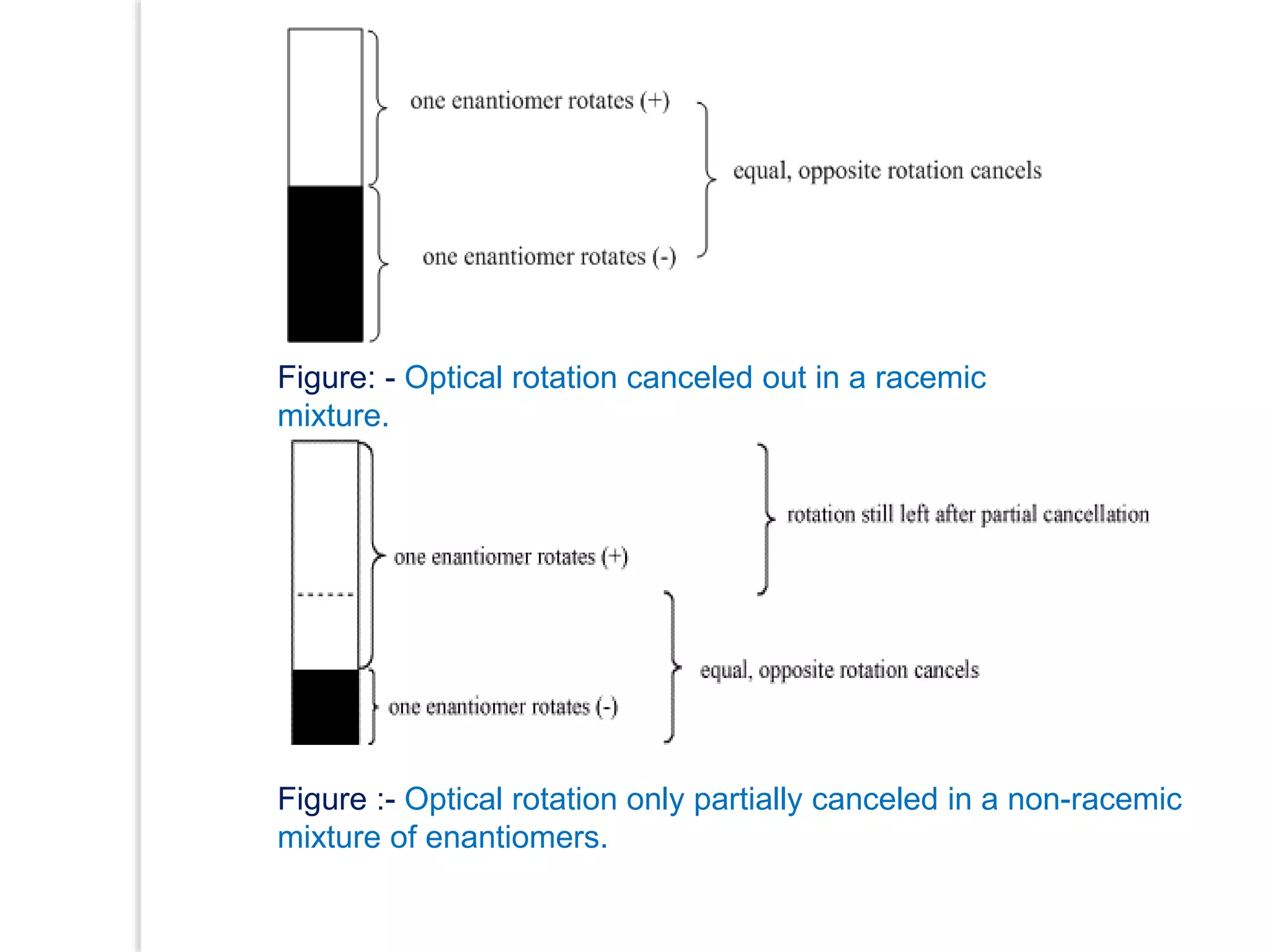
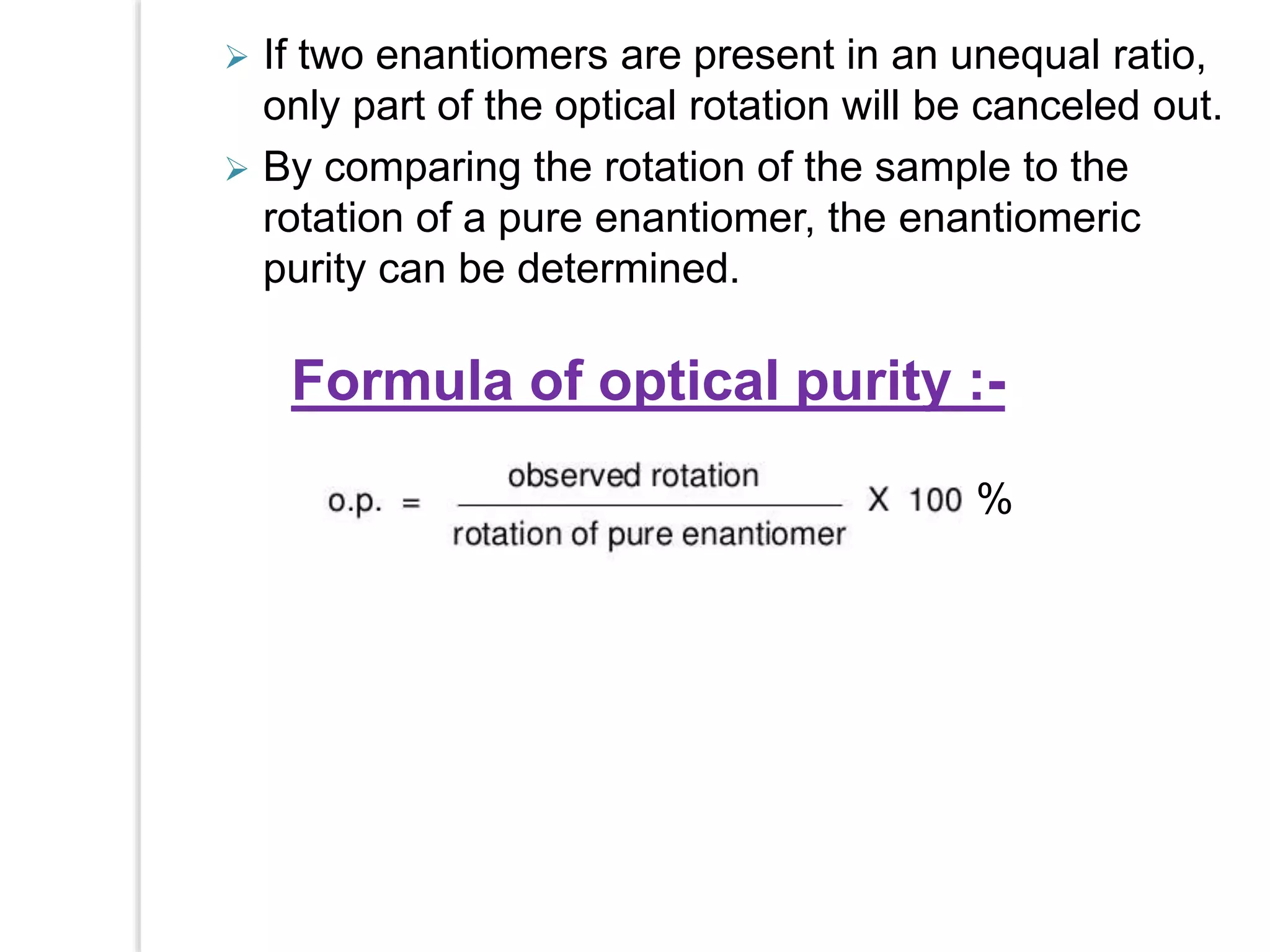
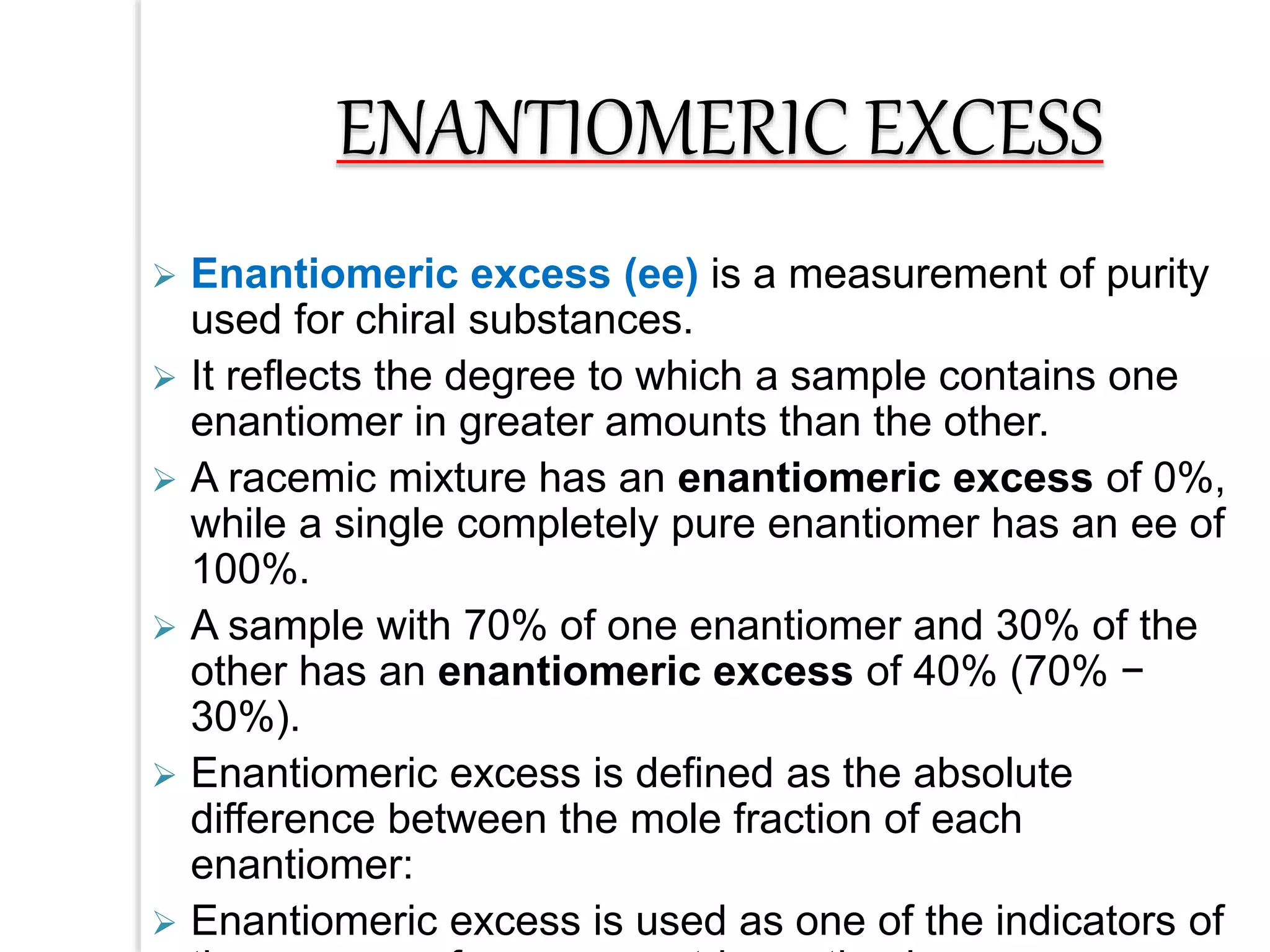
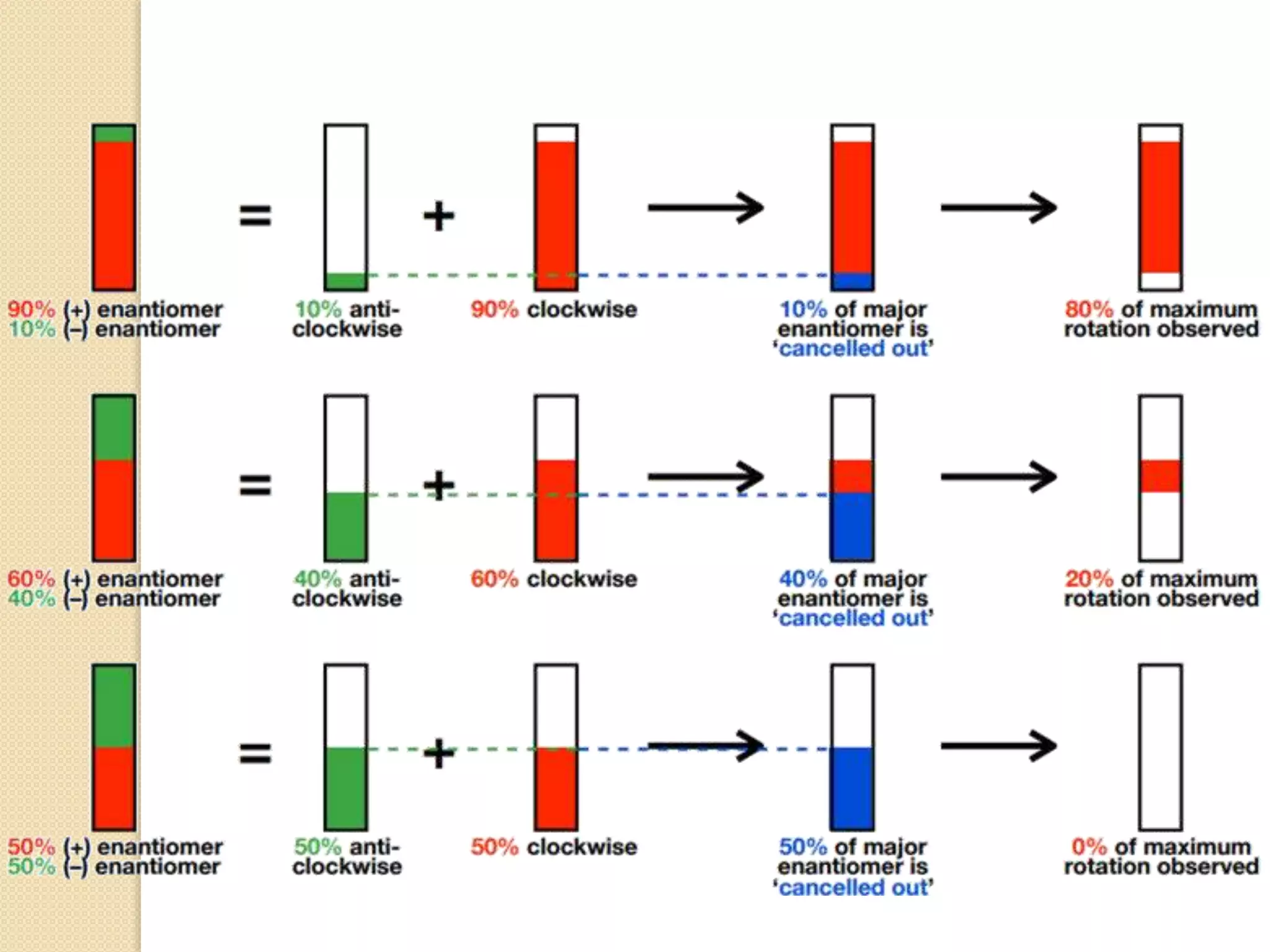
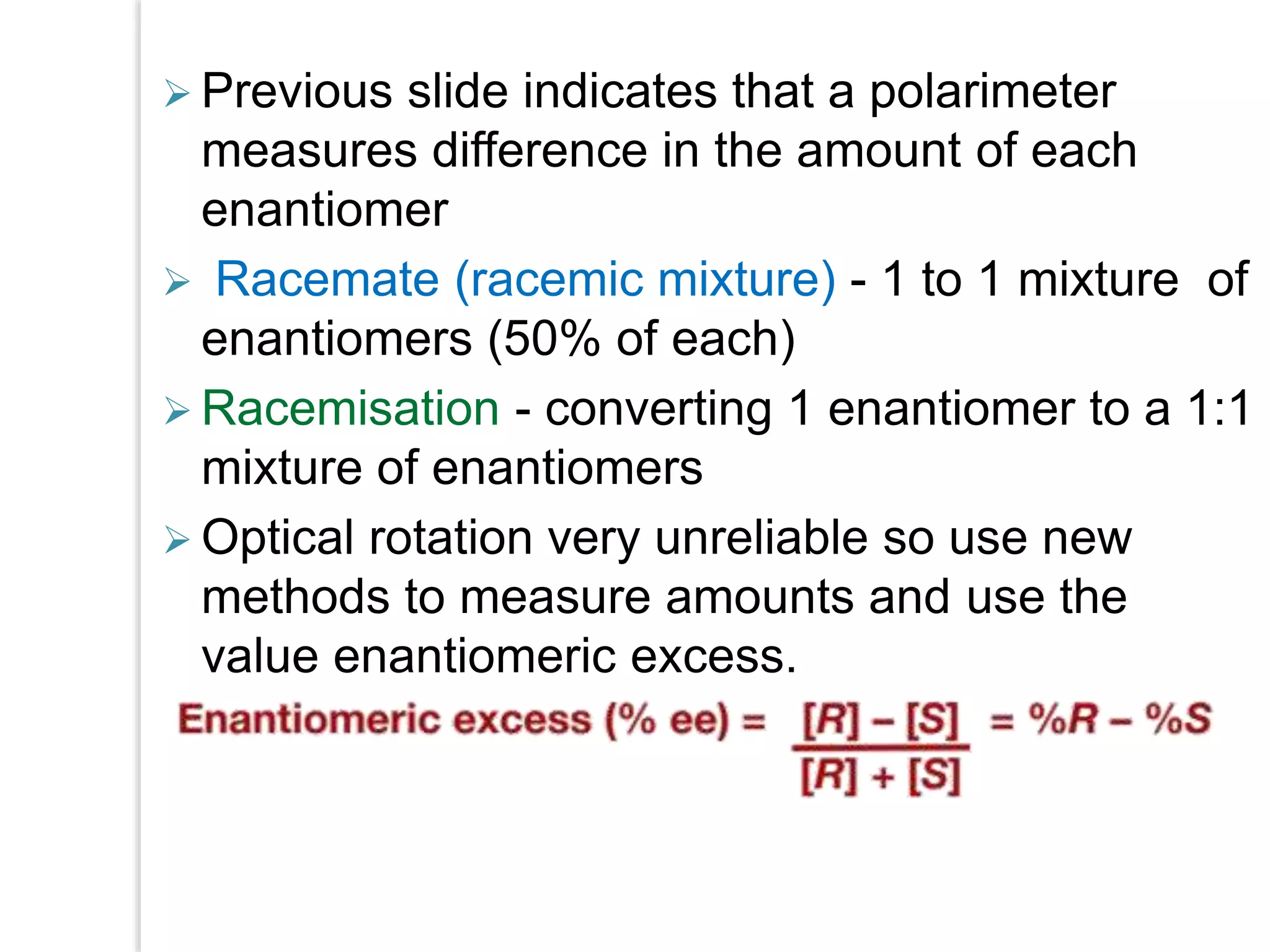
Stereospecific reactions produce different stereoisomeric products from different stereoisomeric starting materials. Stereoselective reactions preferentially form one stereoisomer over others. Examples include the addition of bromine to cis-2-butene, which gives a racemic mixture, whereas addition to trans-2-butene gives a meso diastereomer. Stereoselectivity can be achieved through kinetic or thermodynamic control. Enantioselectivity involves preferential attack on one enantiotopic face, while diastereoselectivity arises from steric hindrance. Optical purity and enantiomeric excess quantify the amounts of each enantiomer present.