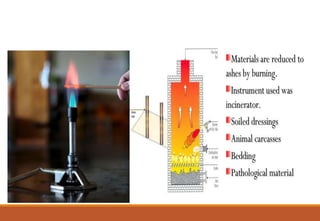
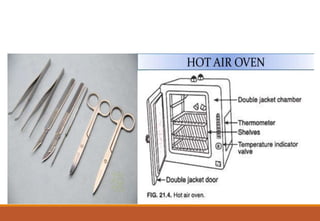
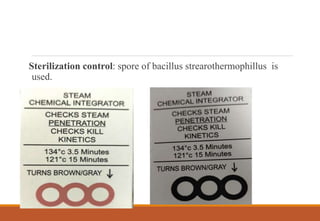
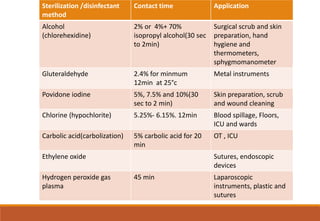
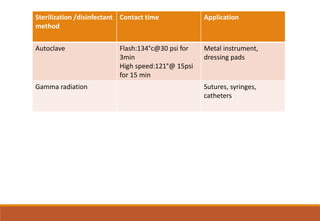
Sterilization is a process that eliminates all microorganisms, while disinfection only eliminates pathogenic or disease-causing ones. Physical sterilization methods include heat, radiation, filtration and drying. Moist heat using an autoclave is the most reliable sterilization method. Chemical agents like alcohol, aldehydes, halogens and phenols are also used for sterilization and disinfection. Proper cleaning is important before sterilization or disinfection. Sterilized items are classified based on the risk of infection if contaminated.