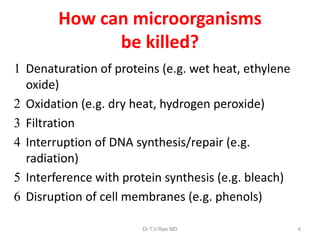
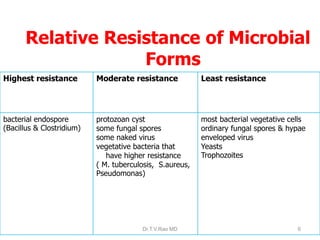
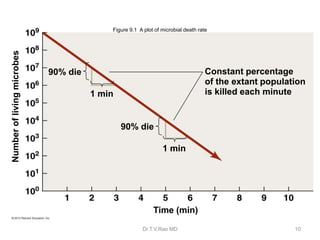
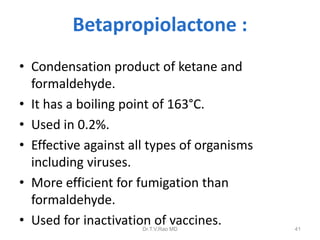
This document discusses sterilization and disinfection using chemicals. It begins by explaining why sterilization is needed to remove or destroy microorganisms that can cause infection. It then discusses various chemical methods used for sterilization and disinfection, including alcohols, aldehydes, phenols, halogens, oxidizing agents, salts, and others. It provides details on specific chemicals like formaldehyde, glutaraldehyde, chlorhexidine, iodine, and hydrogen peroxide. It also covers factors that influence the efficacy of disinfection like contact time, temperature, and type of microorganism. The document aims to provide information on proper sterilization and disinfection using chemical methods.