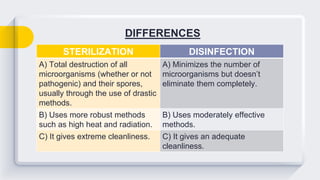
The document discusses chemical disinfection, defining it as the process of eliminating pathogenic organisms on inert surfaces, contrasting it with sterilization. It details various chemical disinfectants, their modes of action, ideal characteristics, potency levels, and specific agents including alcohols, aldehydes, and halogens. The text also outlines the uses, limitations, and effectiveness of these disinfectants in medical and healthcare settings.