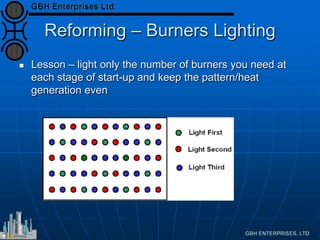
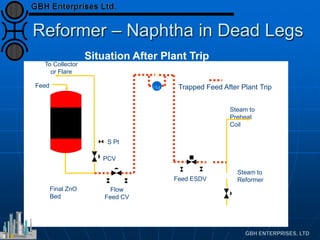
This document presents an analysis of catalyst mal-operation incidents in syngas production, providing recommendations for plant operators to avoid costly outages and catalyst losses. It covers topics such as catalyst loading, burner operation during start-up, carbon formation from hydrocarbons, and failures due to condensation. Key lessons emphasize the importance of proper training, catalyst selection, and equipment maintenance to improve operational reliability.