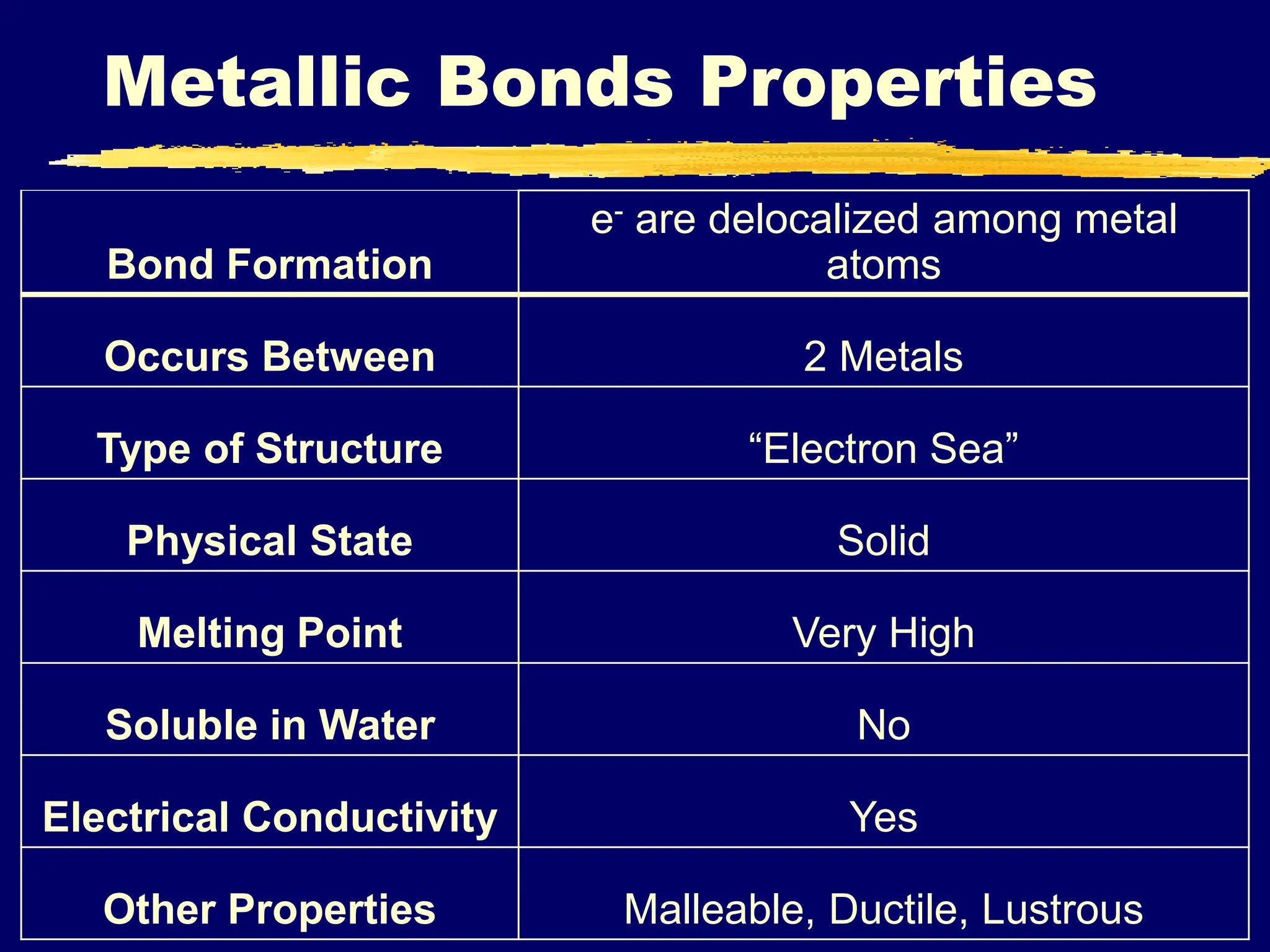
Metallic bonding occurs between metal atoms when electrons are delocalized and shared among the entire structure, forming an "electron sea". This bonding results in properties such as high melting points, conductivity of heat and electricity, malleability, ductility, and a solid physical state. Metallic bonding is quite strong due to the attractive force between the positively charged metal ions and the shared electrons.