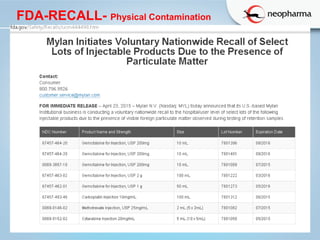
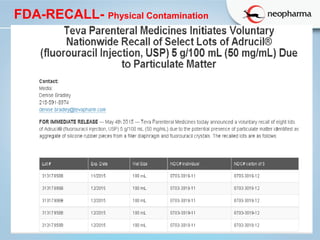
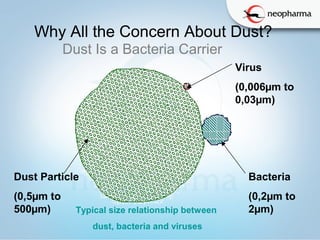
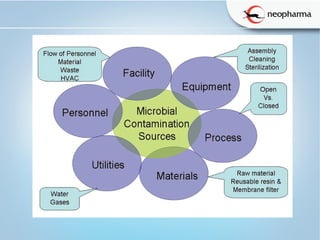
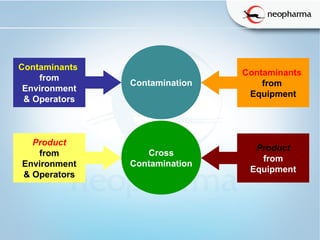
This document discusses microbial contamination and cross-contamination in pharmaceutical manufacturing. It defines cross-contamination as the presence of small quantities of other products manufactured in the same premises. The types of contamination are physical, chemical, and biological from sources like personnel, equipment, and environment. Cross-contamination can be minimized through personnel procedures, adequate facilities, closed production systems, cleaning validation, and proper air pressure differentials. Special attention needs to be given to prevent cross-contamination of beta-lactam drugs like penicillin which can cause allergic reactions in sensitive patients. FDA regulations require dedicated areas for their production to prevent sensitization issues. Personnel hygiene and housekeeping are important to control contamination.