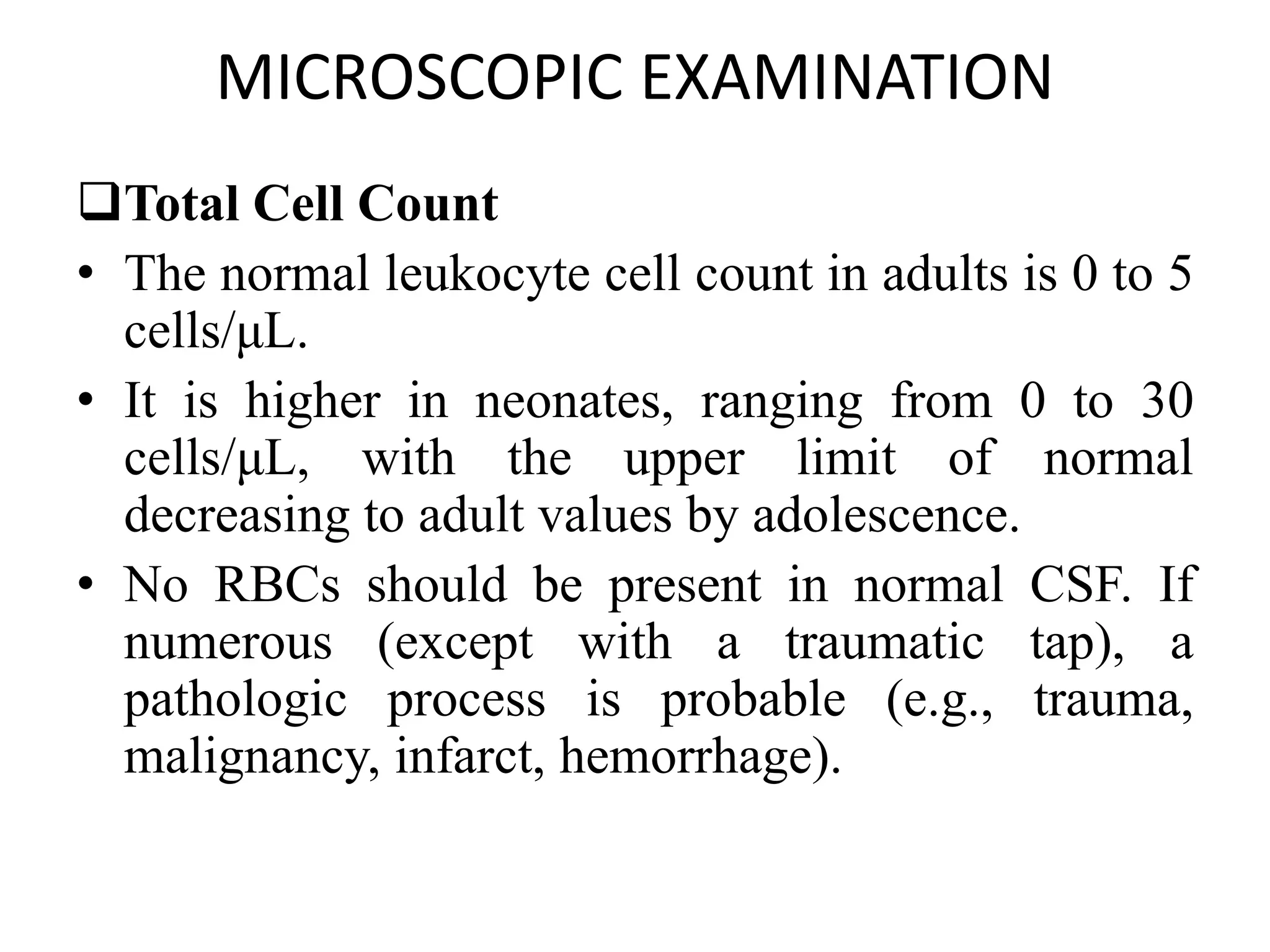
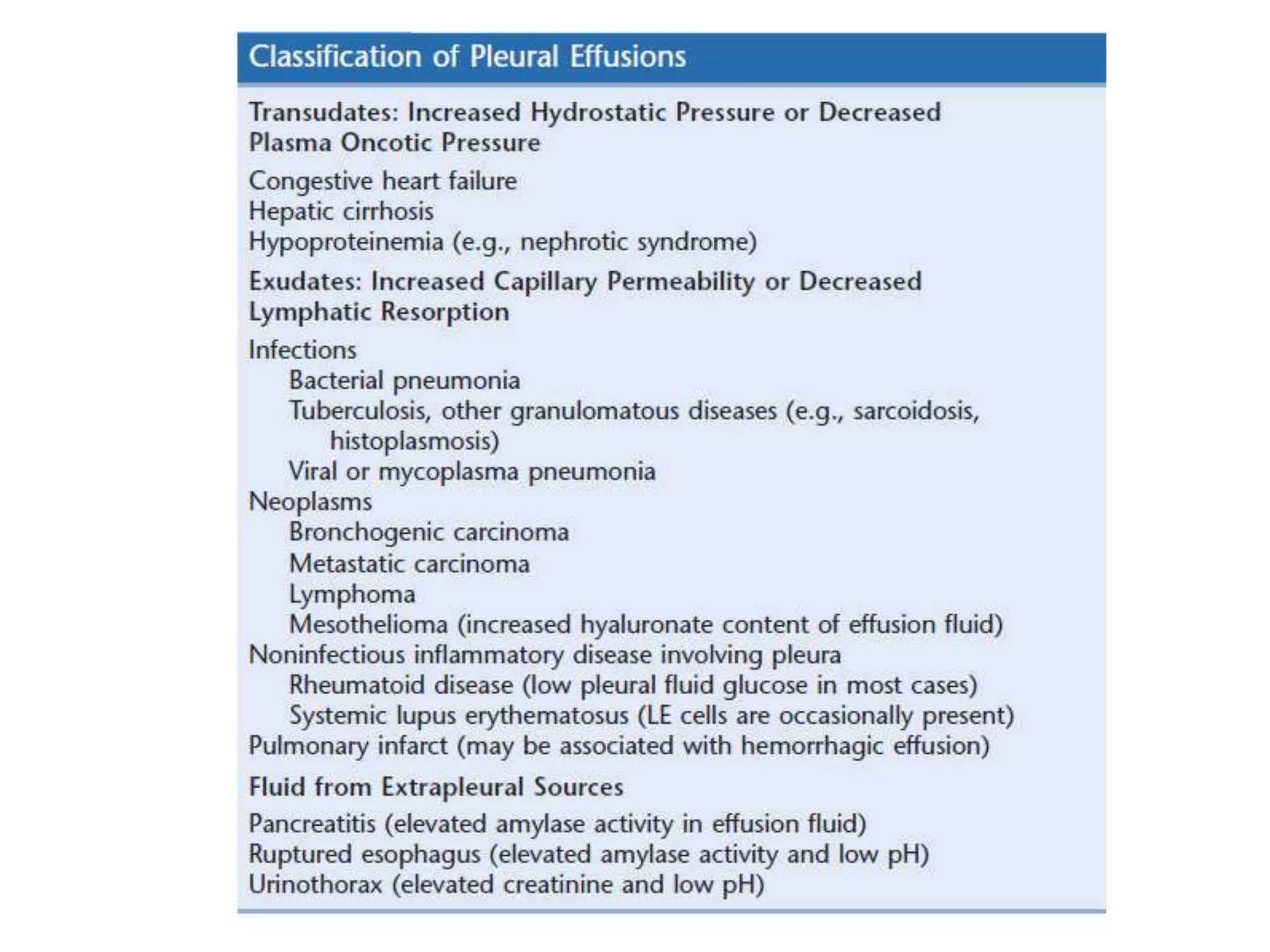
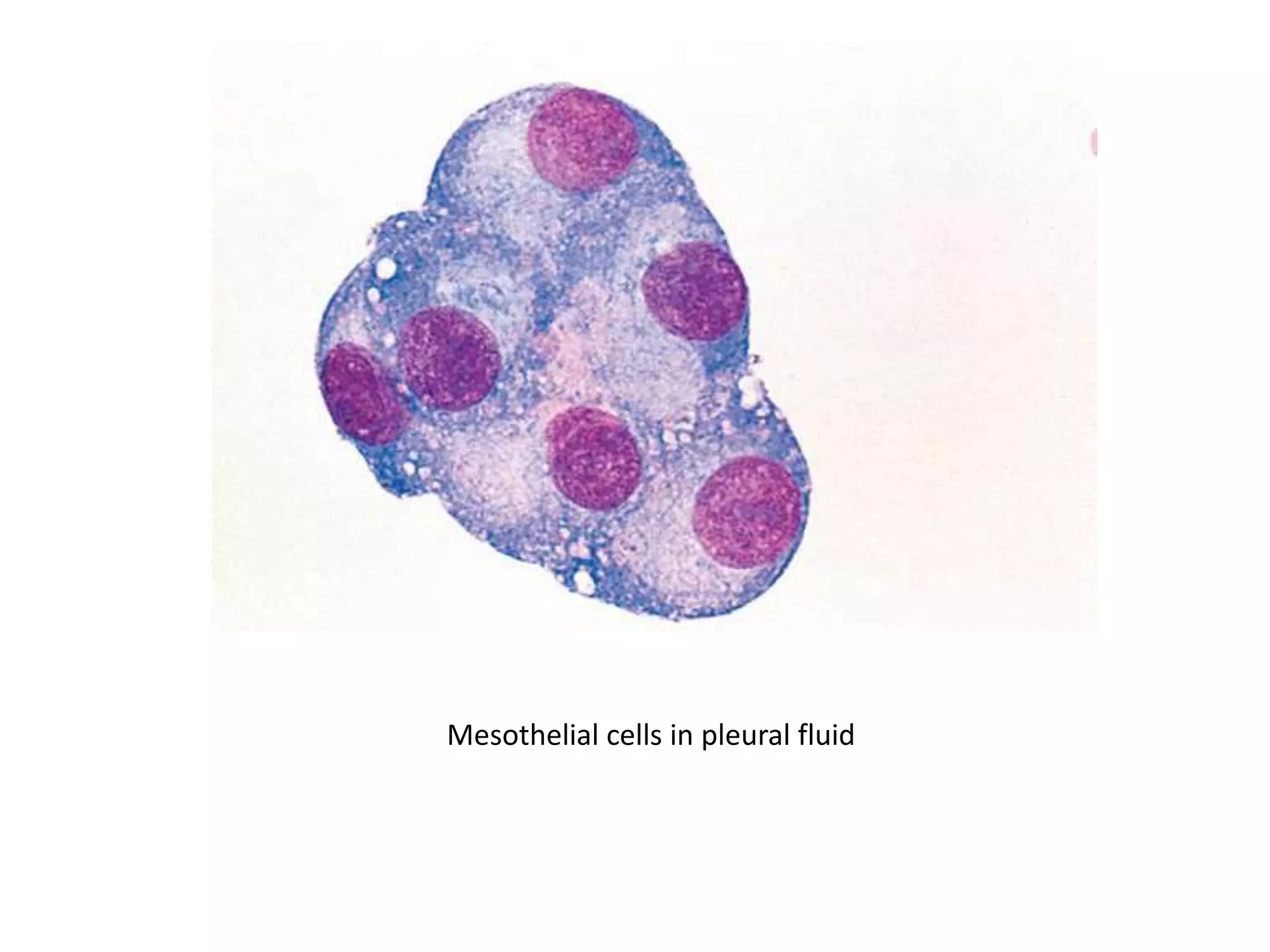
This document summarizes guidelines for examining various body fluids, including cerebrospinal fluid, pleural fluid, peritoneal fluid, and semen. For cerebrospinal fluid, normal volumes and cell counts are provided. Guidelines are given for collection into tubes and microscopic examination, including normal cell differentials. Similar information is provided for examining pleural fluid, peritoneal fluid, and semen, including gross appearance, cell counts, differentials, and factors that affect analysis.