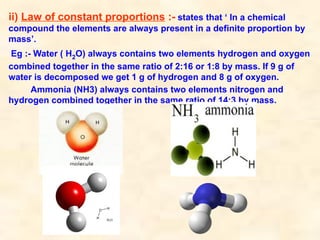
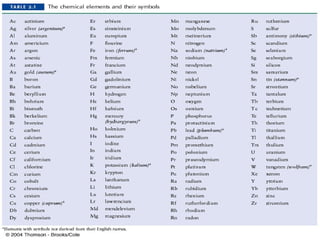
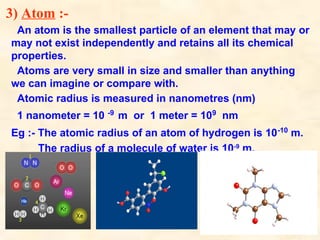
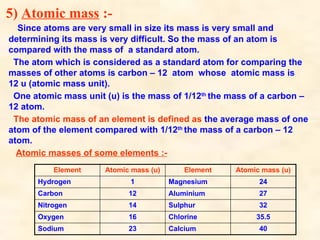
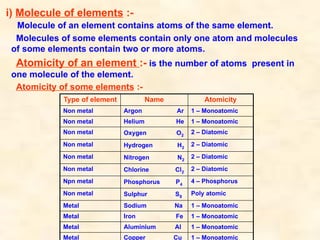
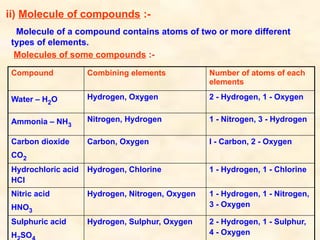
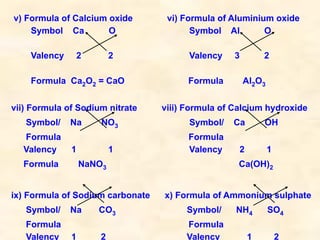
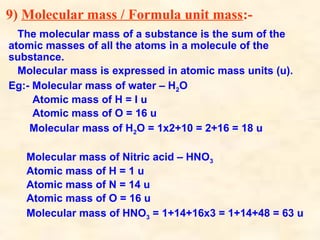
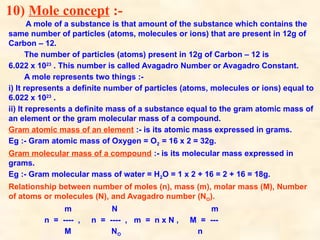
The document discusses the laws of chemical combination, including the law of conservation of mass and the law of constant proportions, outlining key principles of matter and atomic theory. It covers the definitions of atoms, molecules, atomic mass, ions, and the process of writing chemical formulas, along with examples of various elements and compounds. Additionally, it introduces the mole concept, defined as the amount of substance containing Avogadro's number of particles, and its relationship with mass and molar mass.