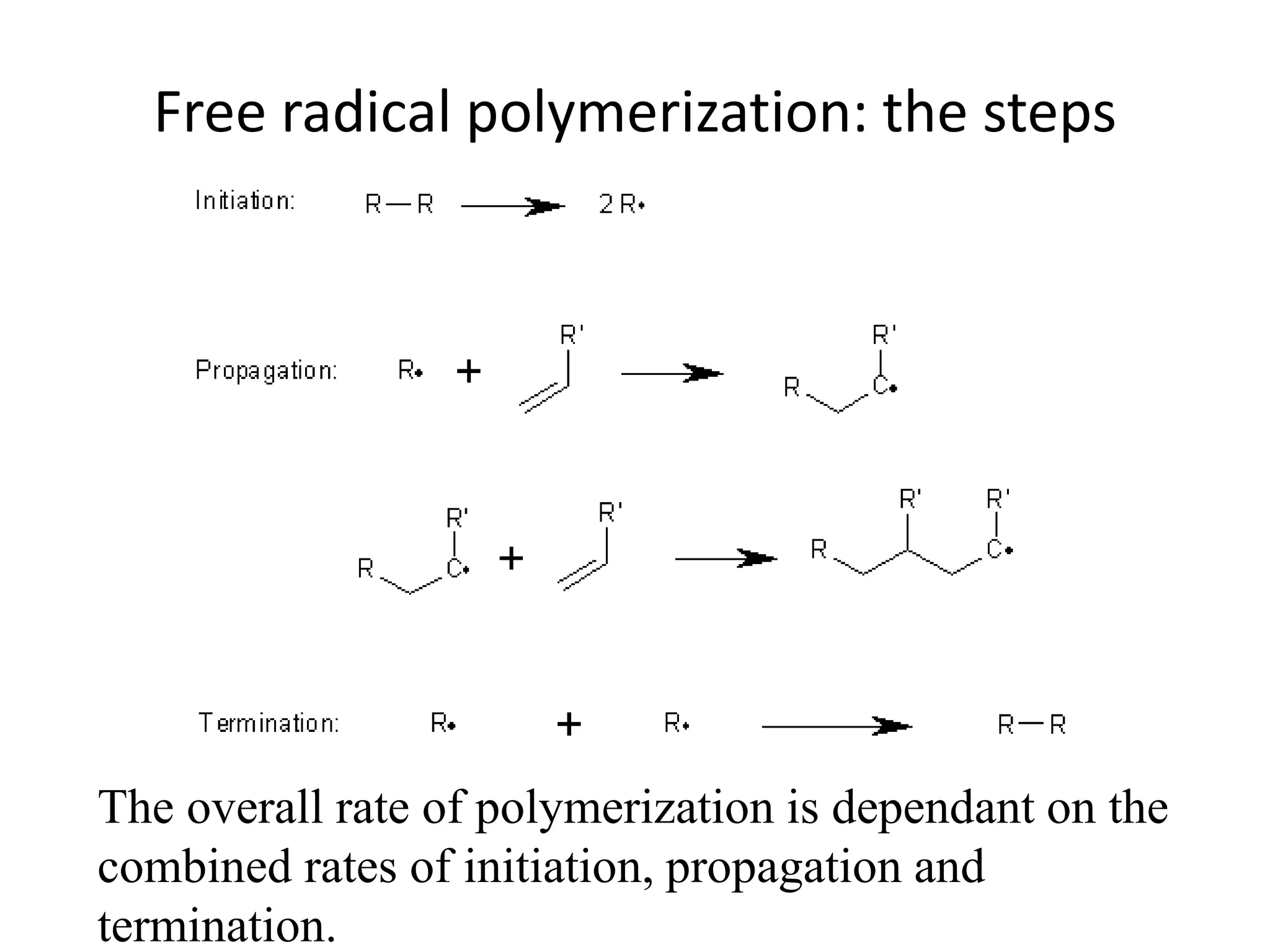
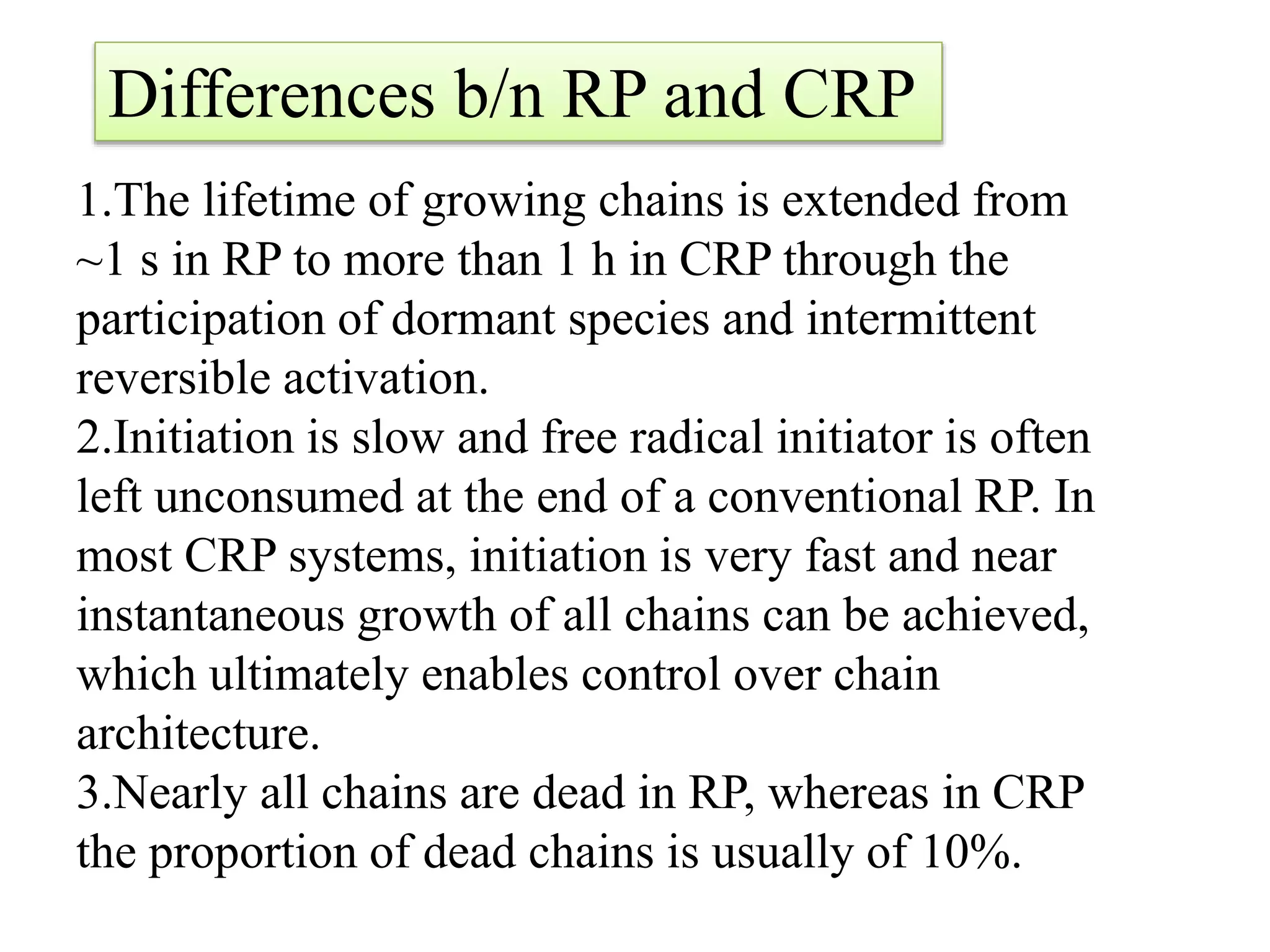
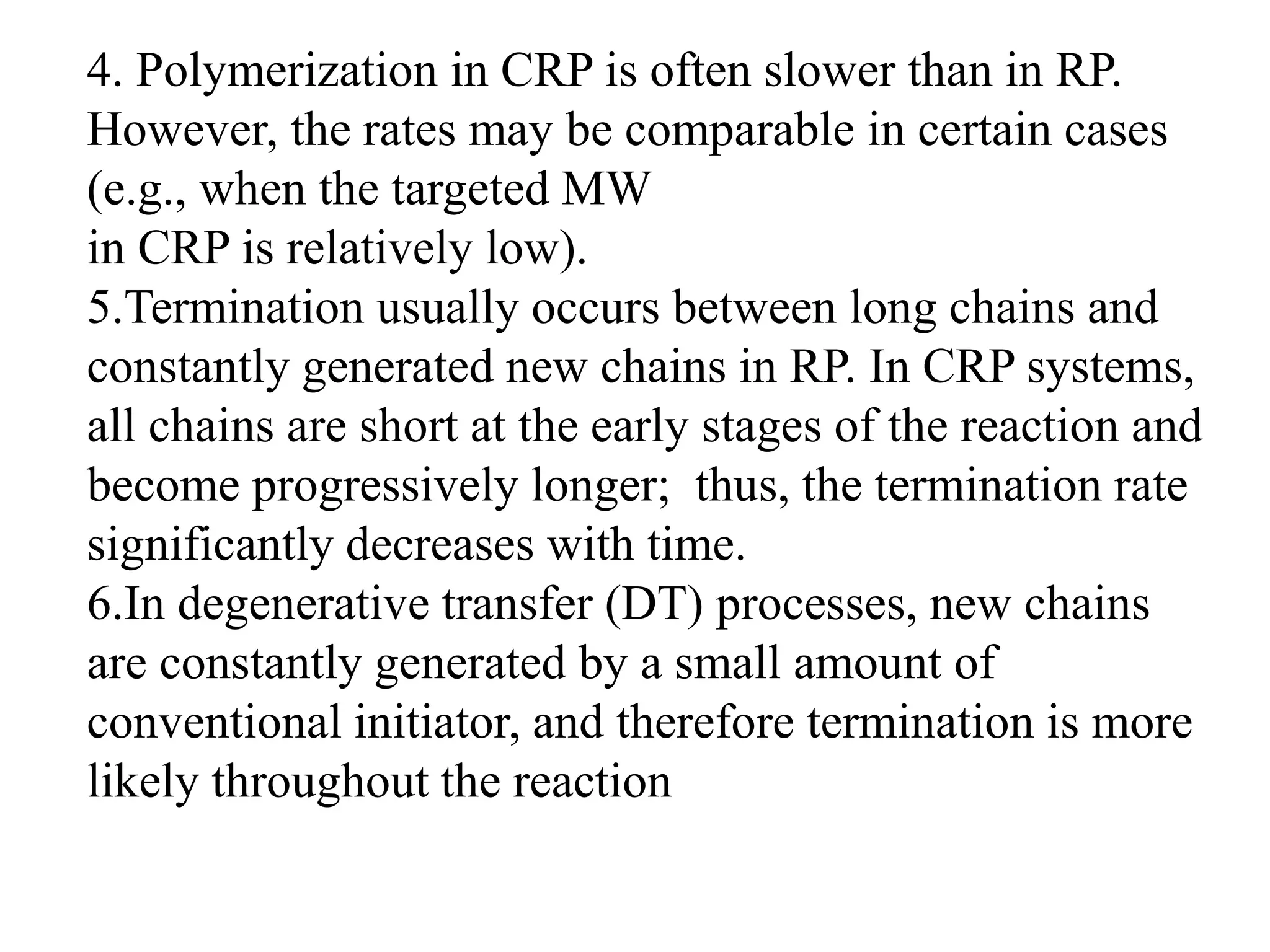
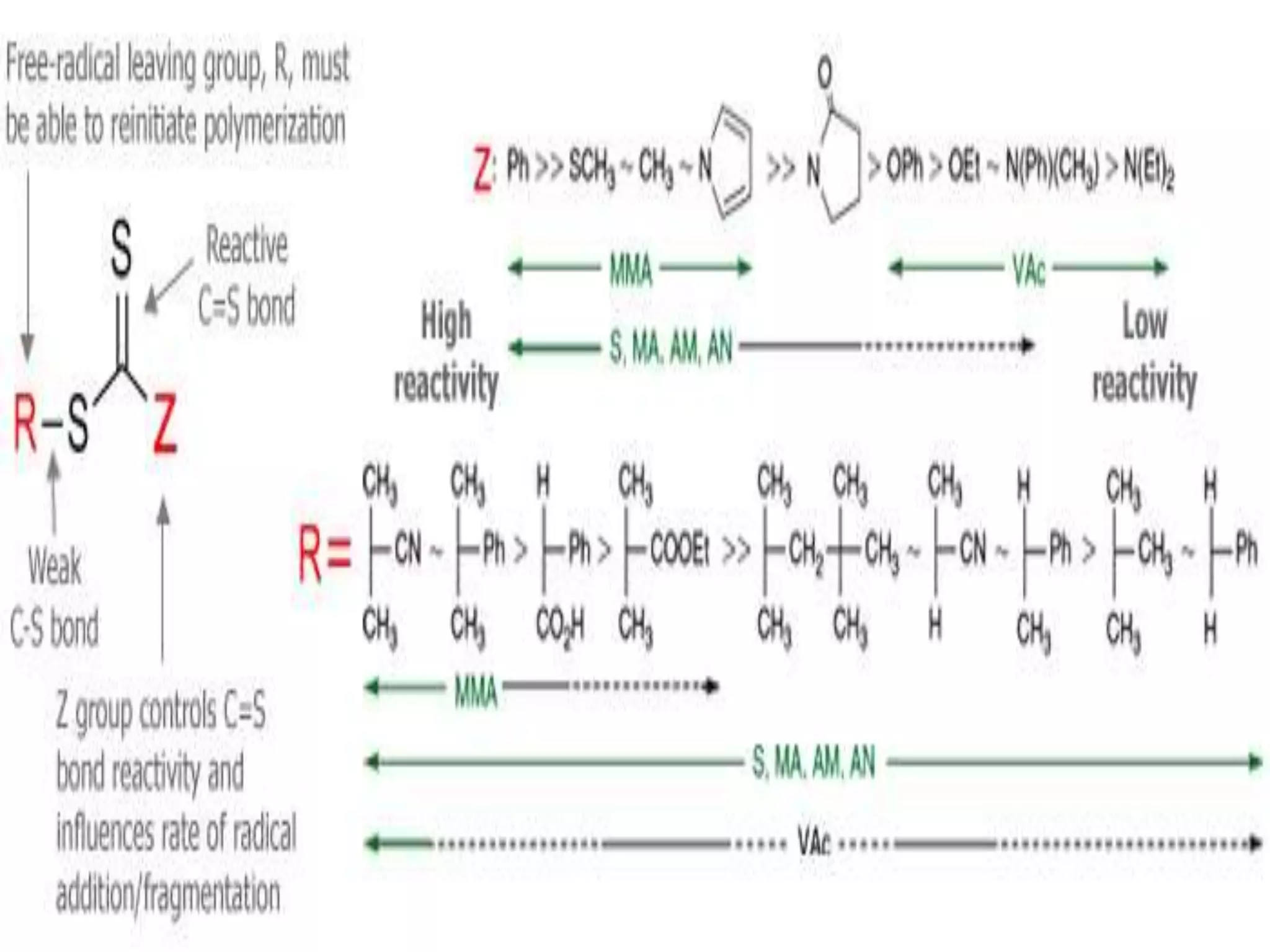
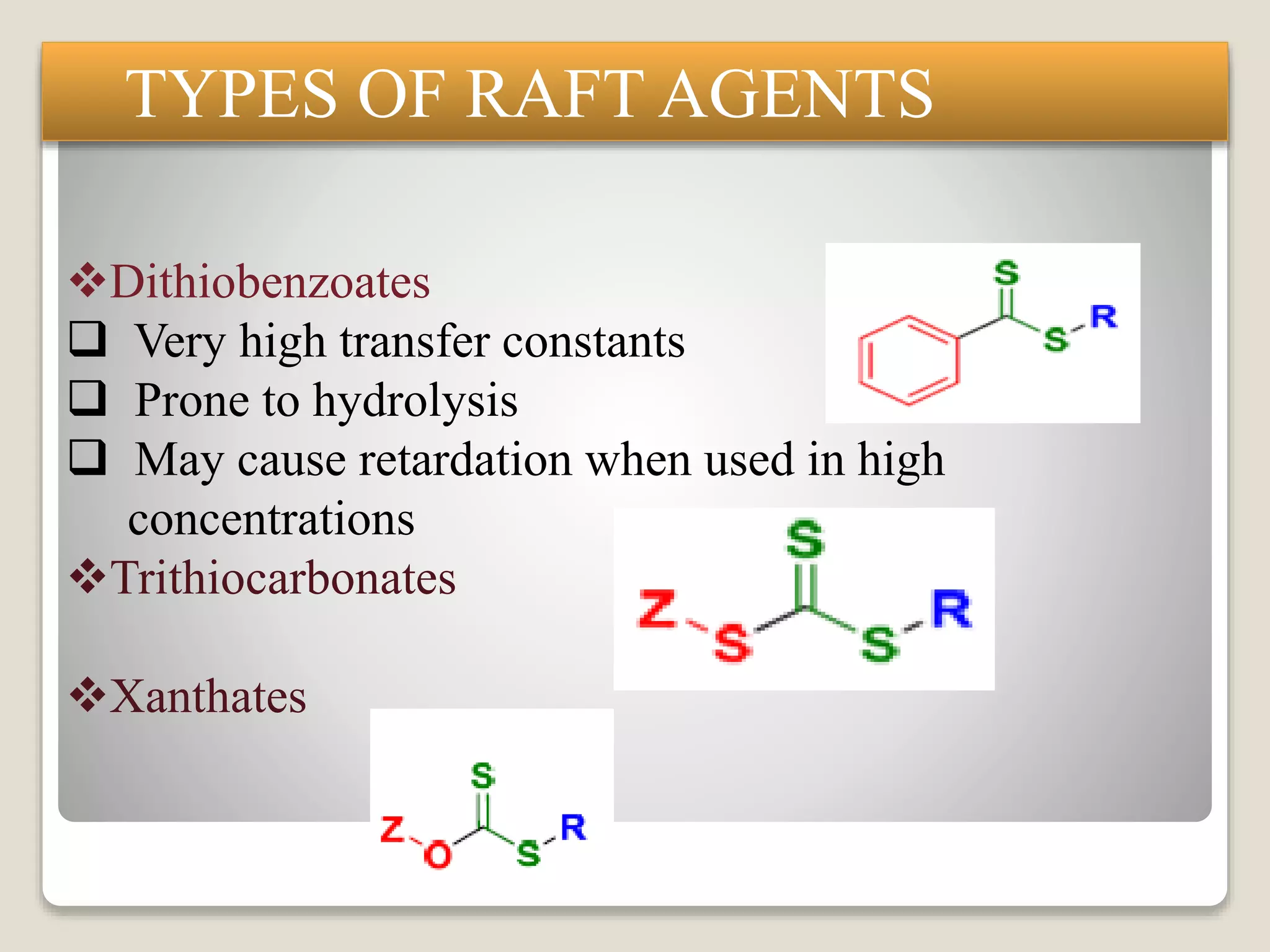
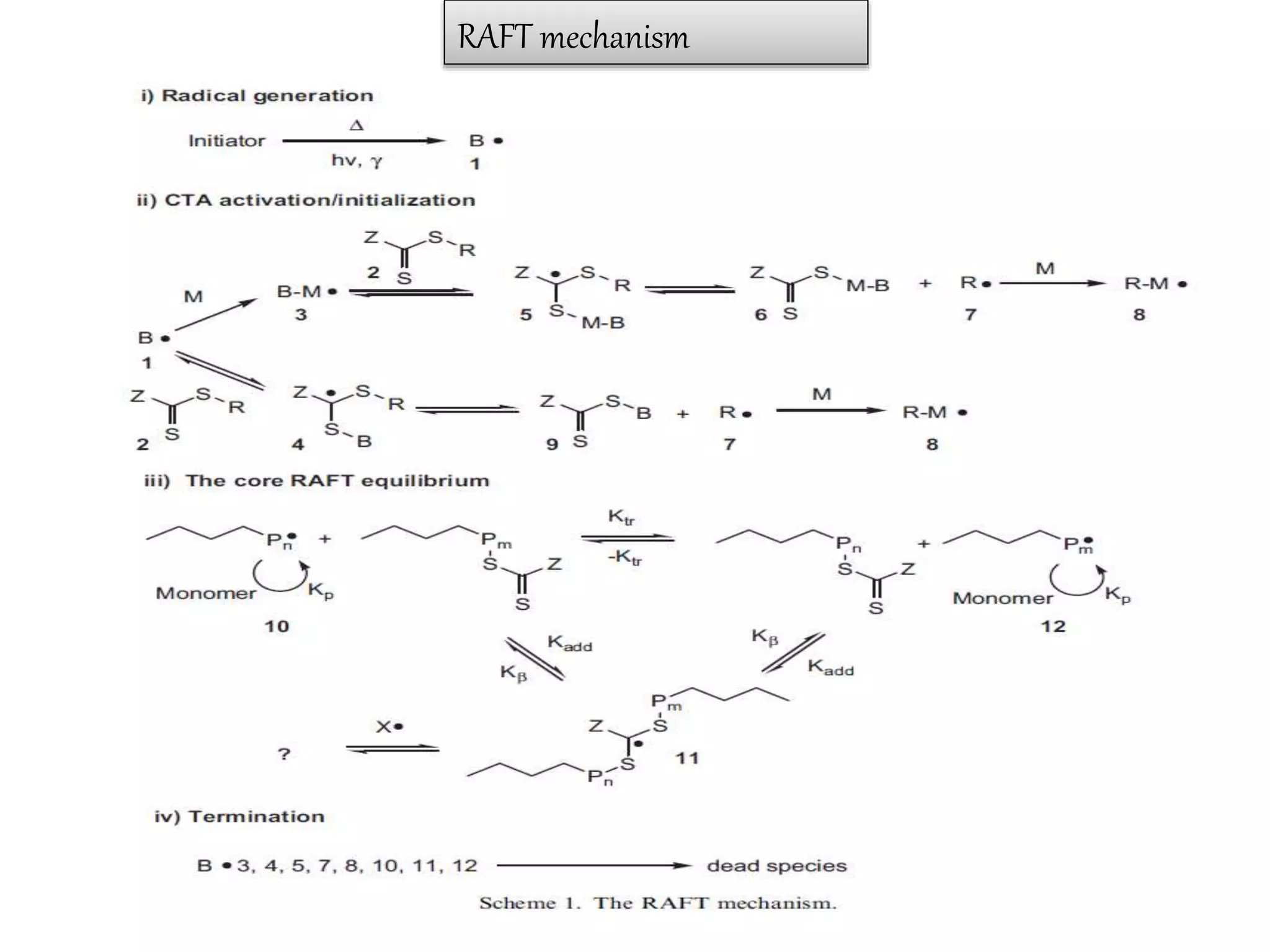
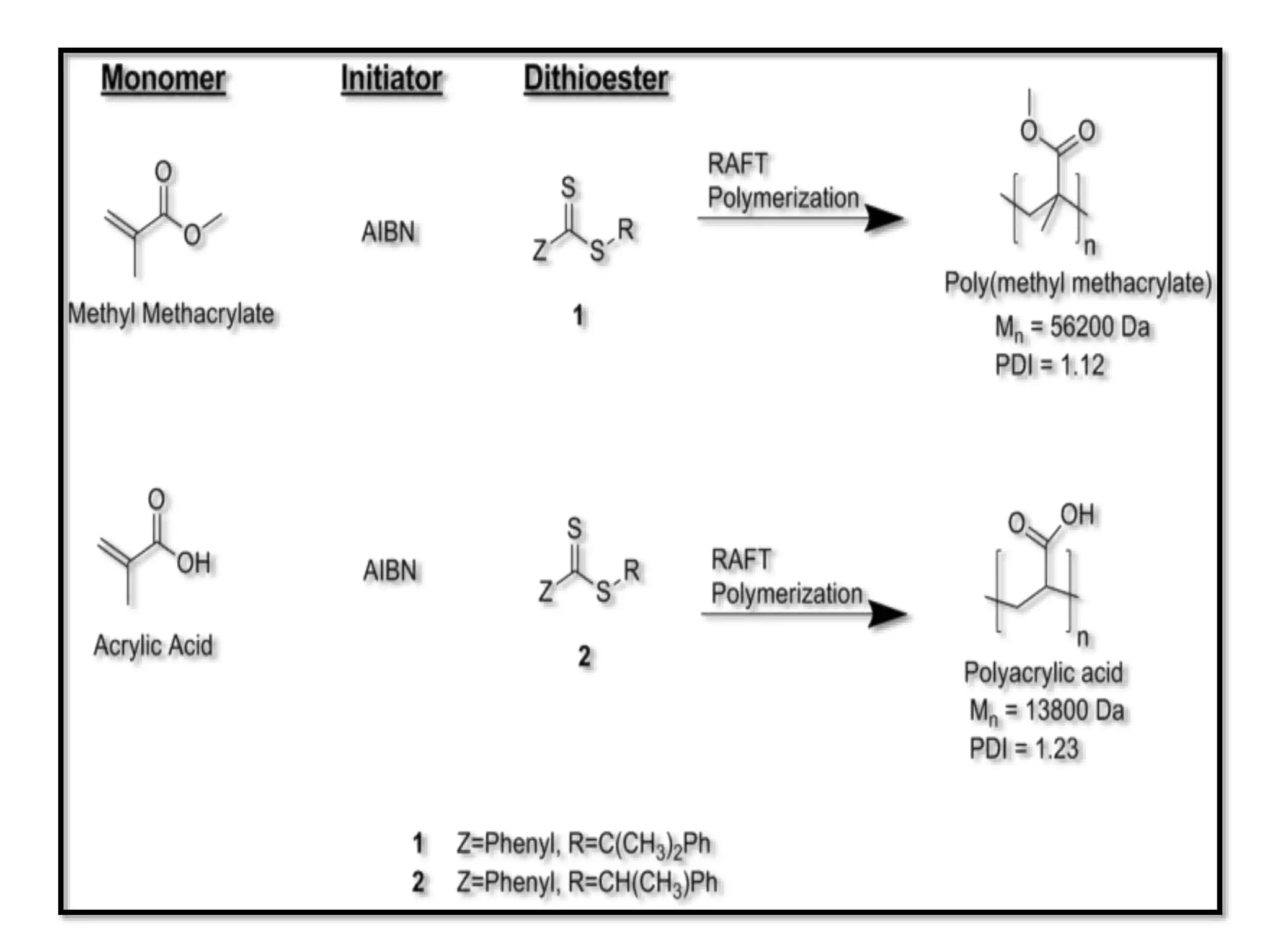
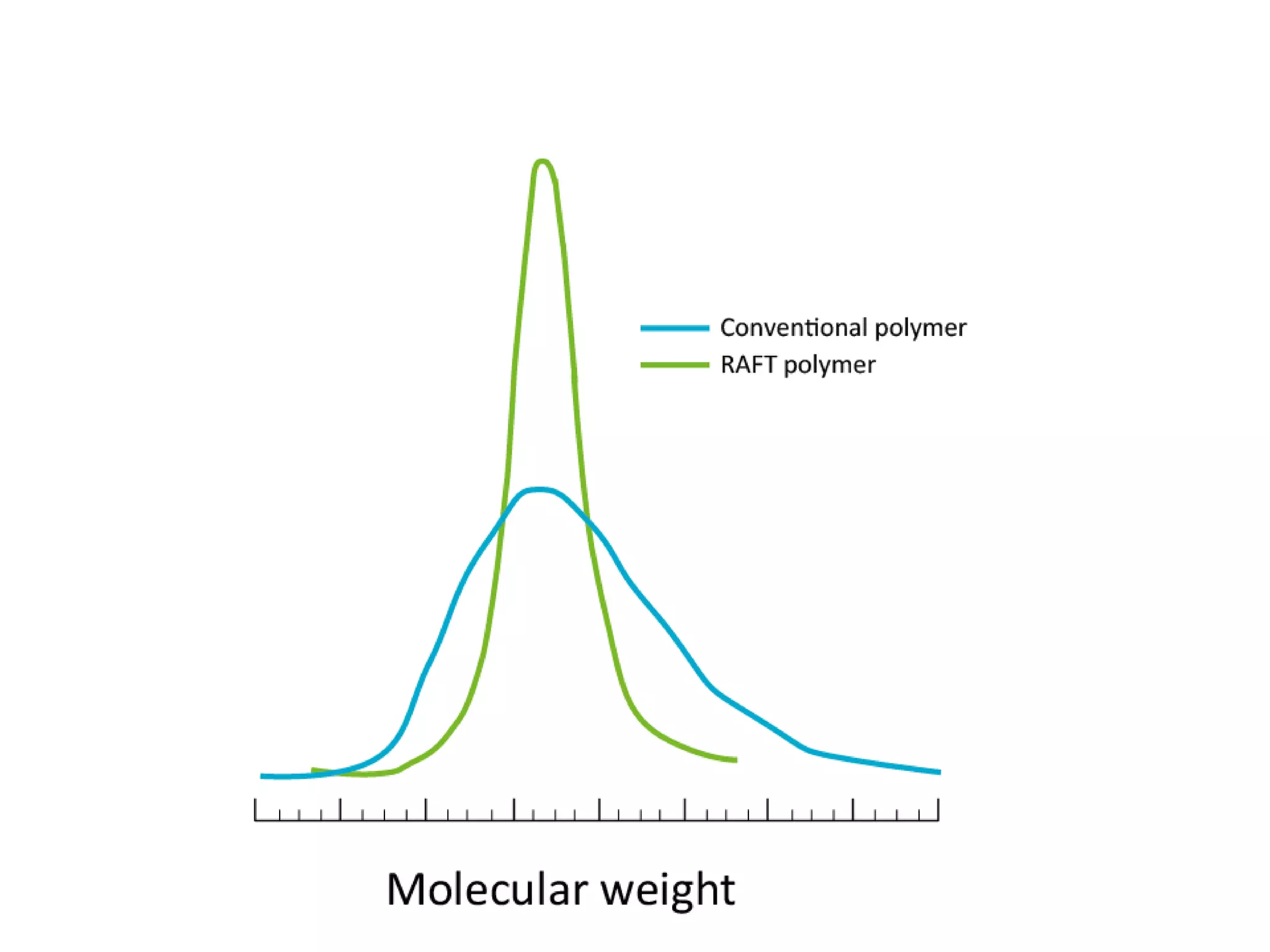
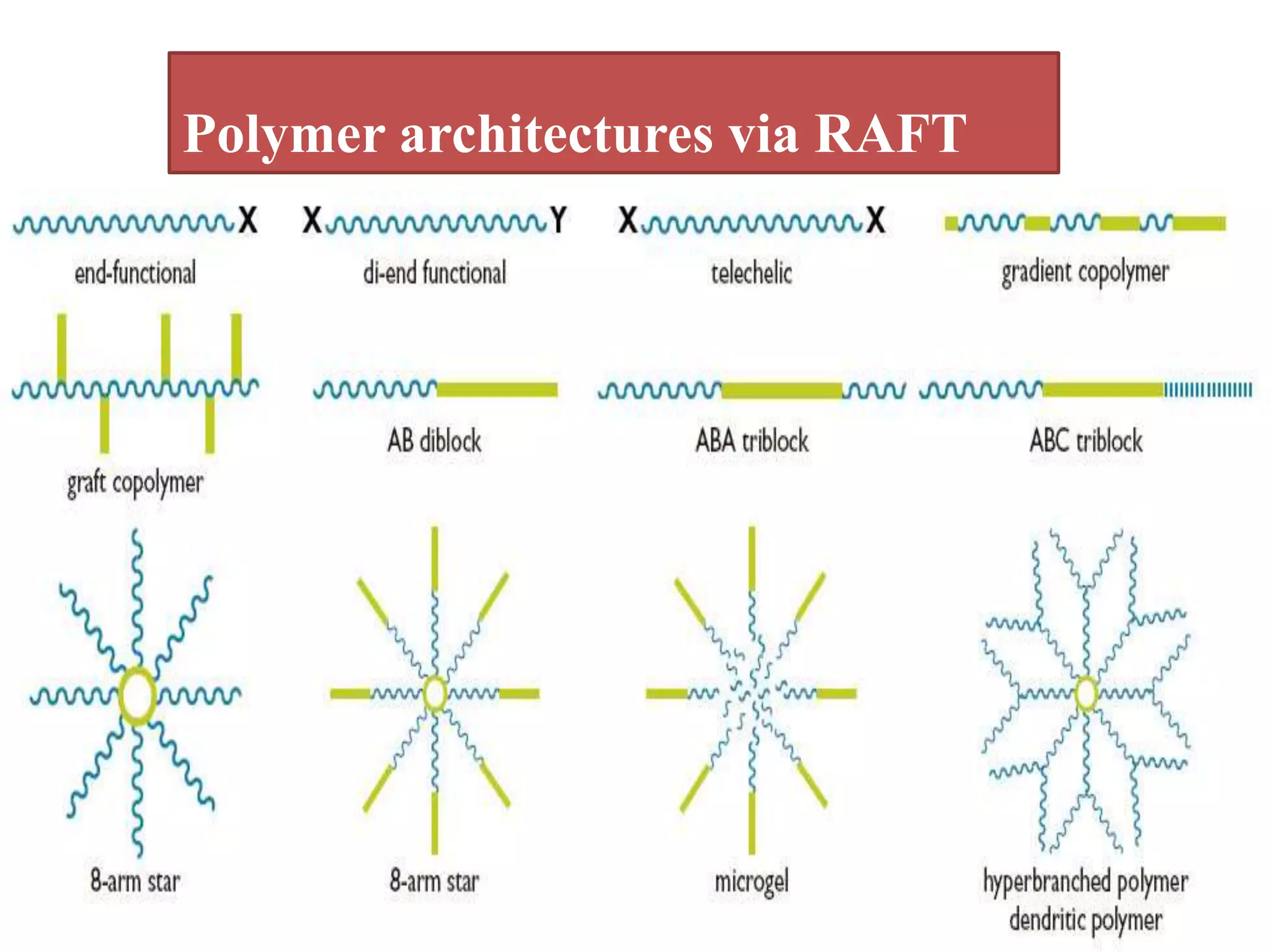
This document provides an overview of controlled radical polymerization (CRP) techniques, with a focus on reversible addition-fragmentation chain transfer (RAFT) polymerization. It compares conventional radical polymerization to CRP, outlines the RAFT mechanism, and discusses outcomes of RAFT including control over molecular weight and architecture. The RAFT process utilizes a RAFT agent during free radical polymerization to extend chain lifetimes and enable living polymer characteristics like well-defined polymers with narrow molecular weight distributions.