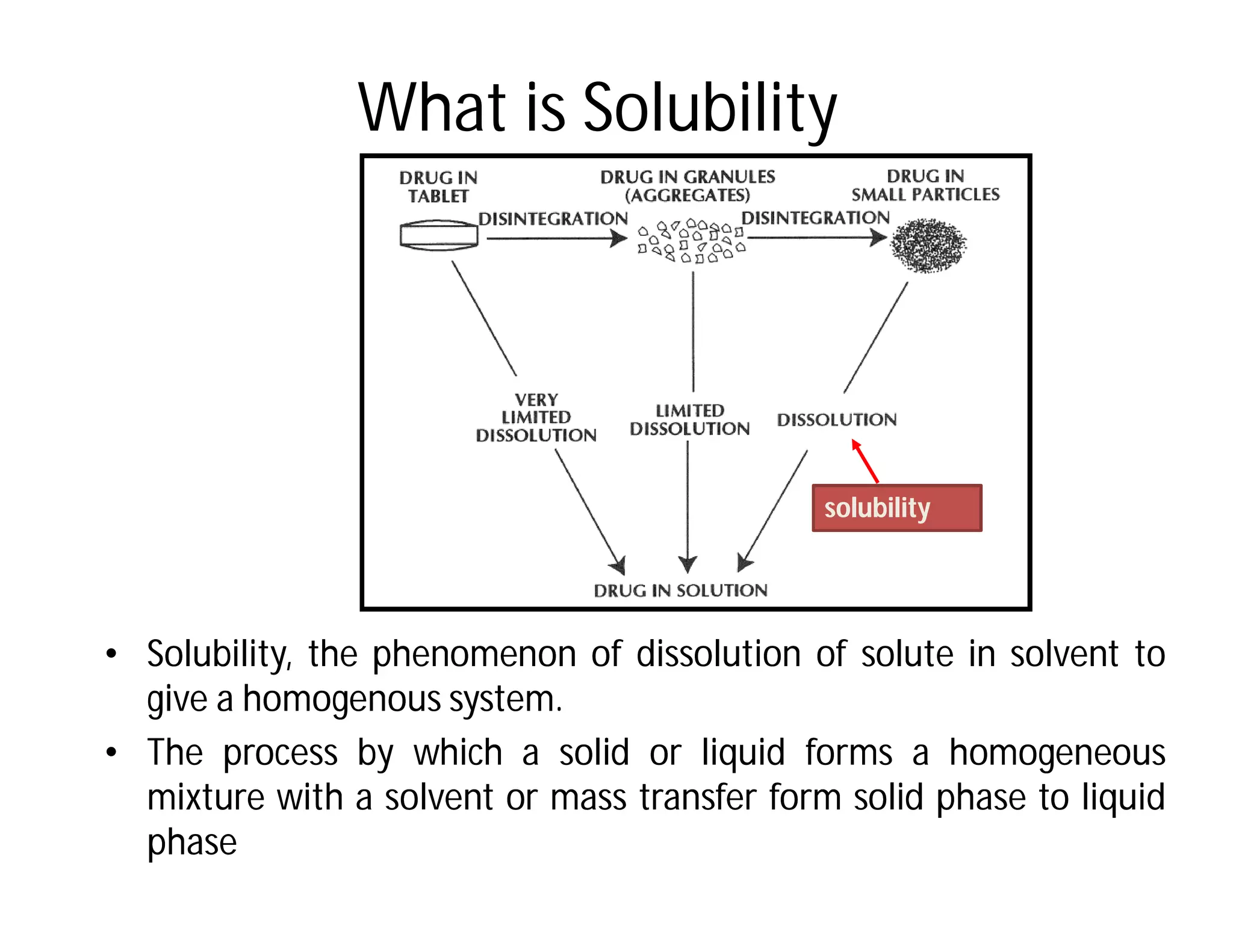
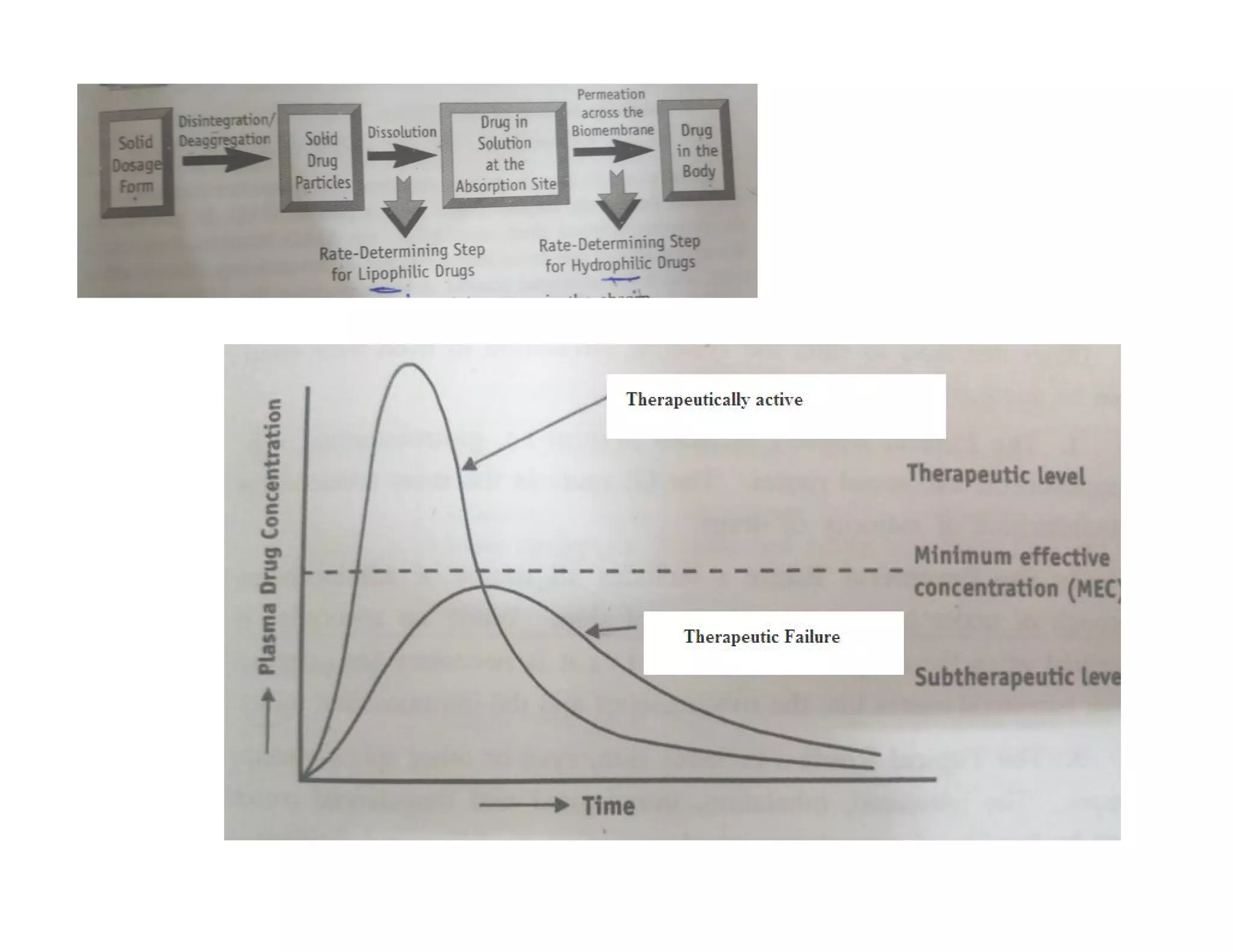
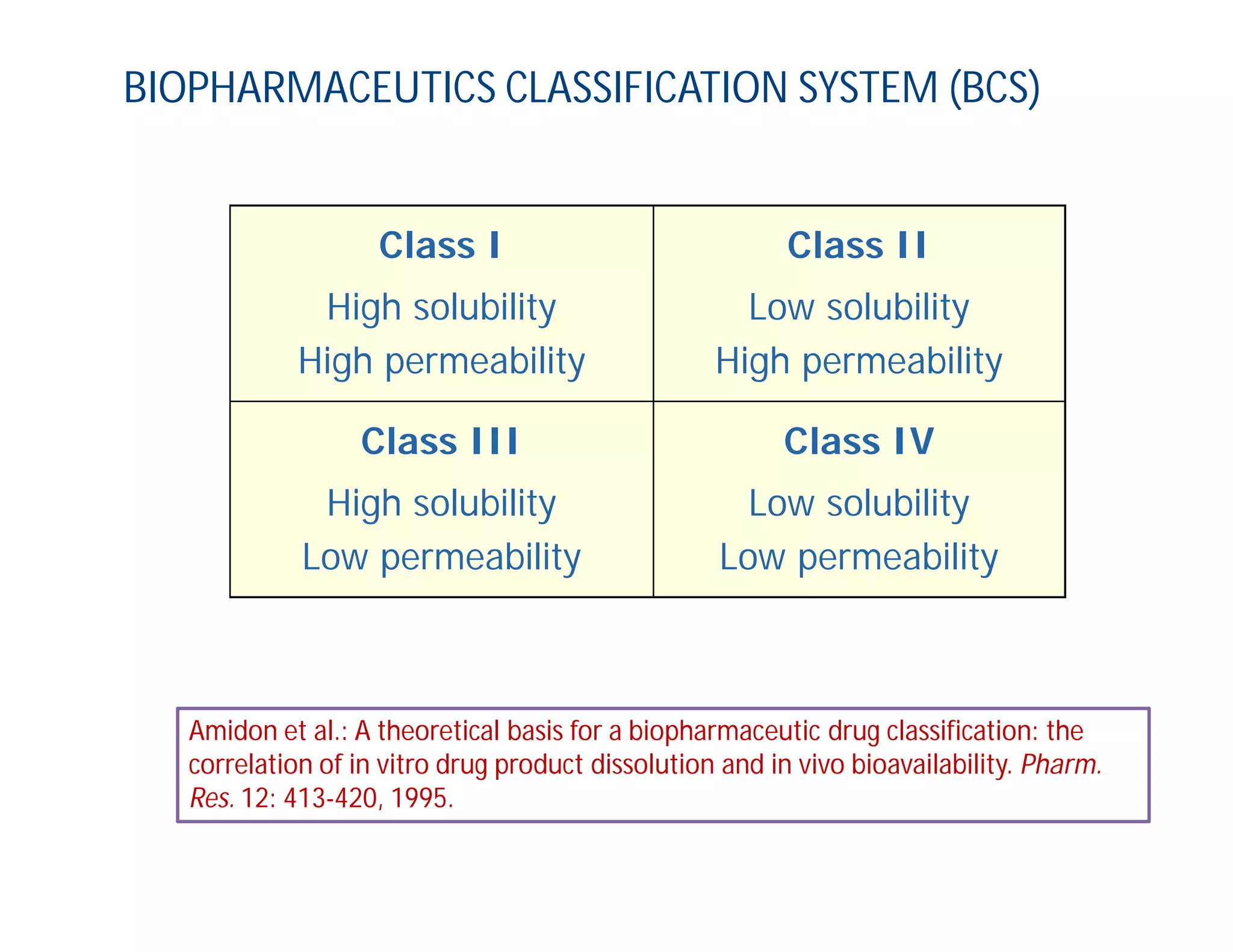
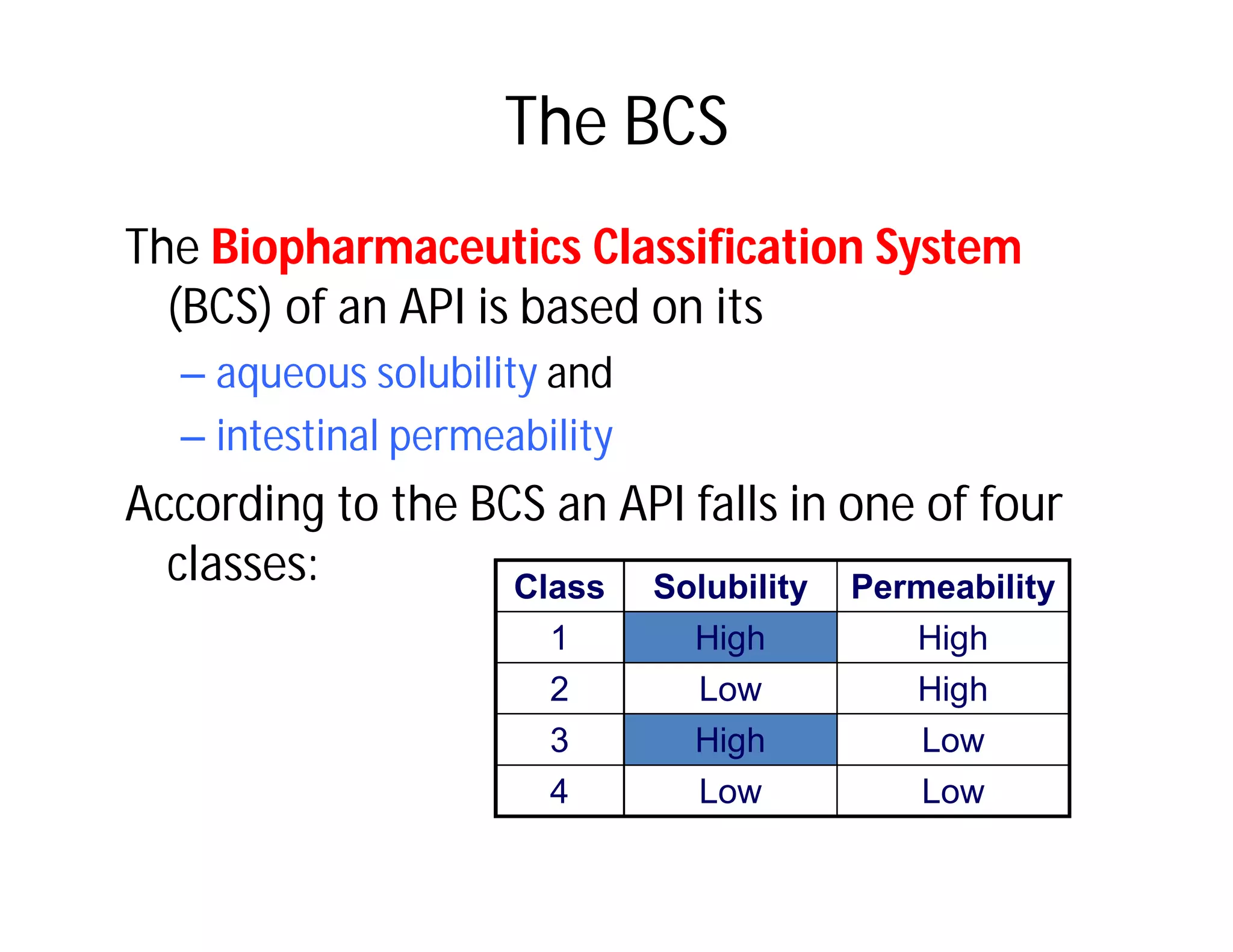
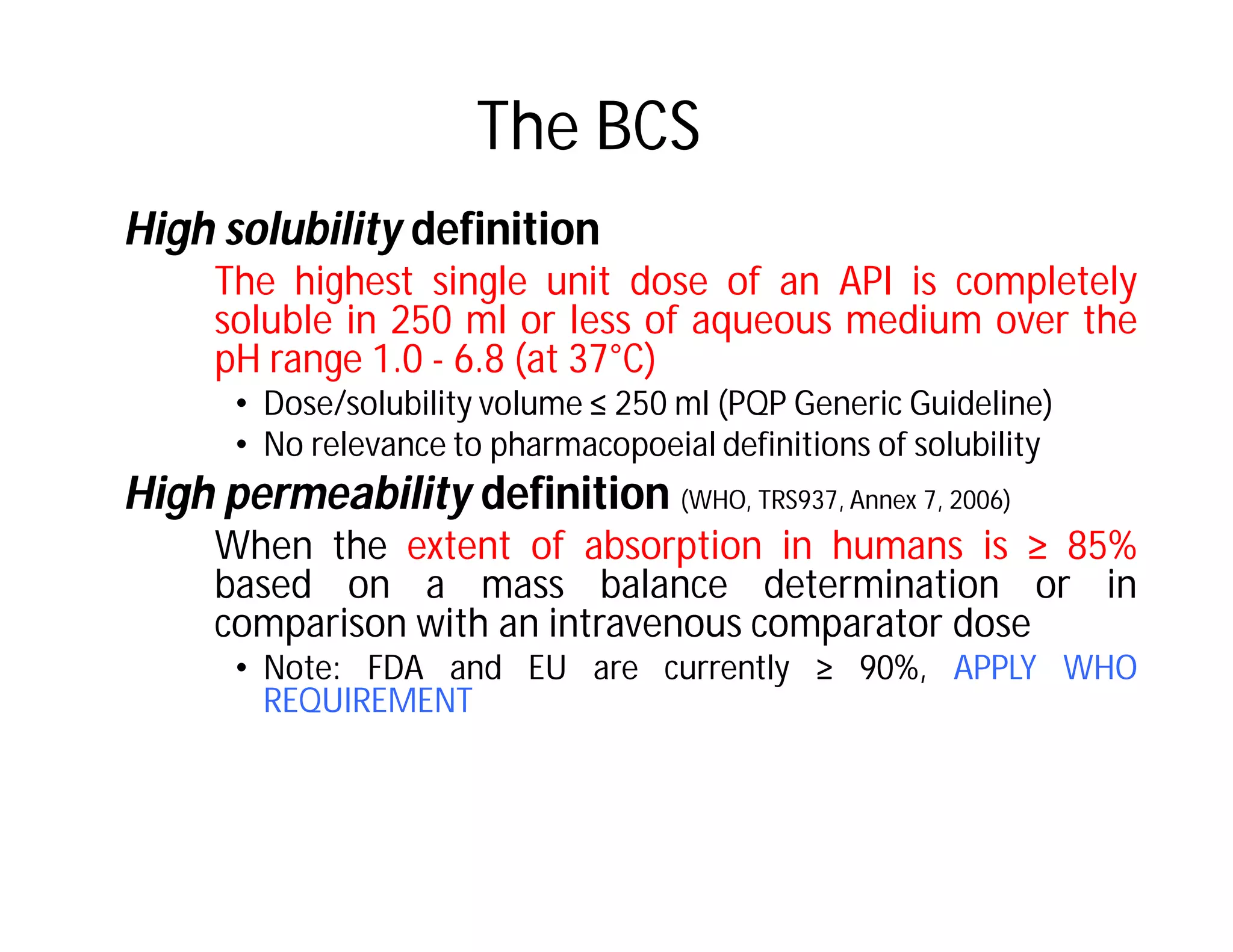
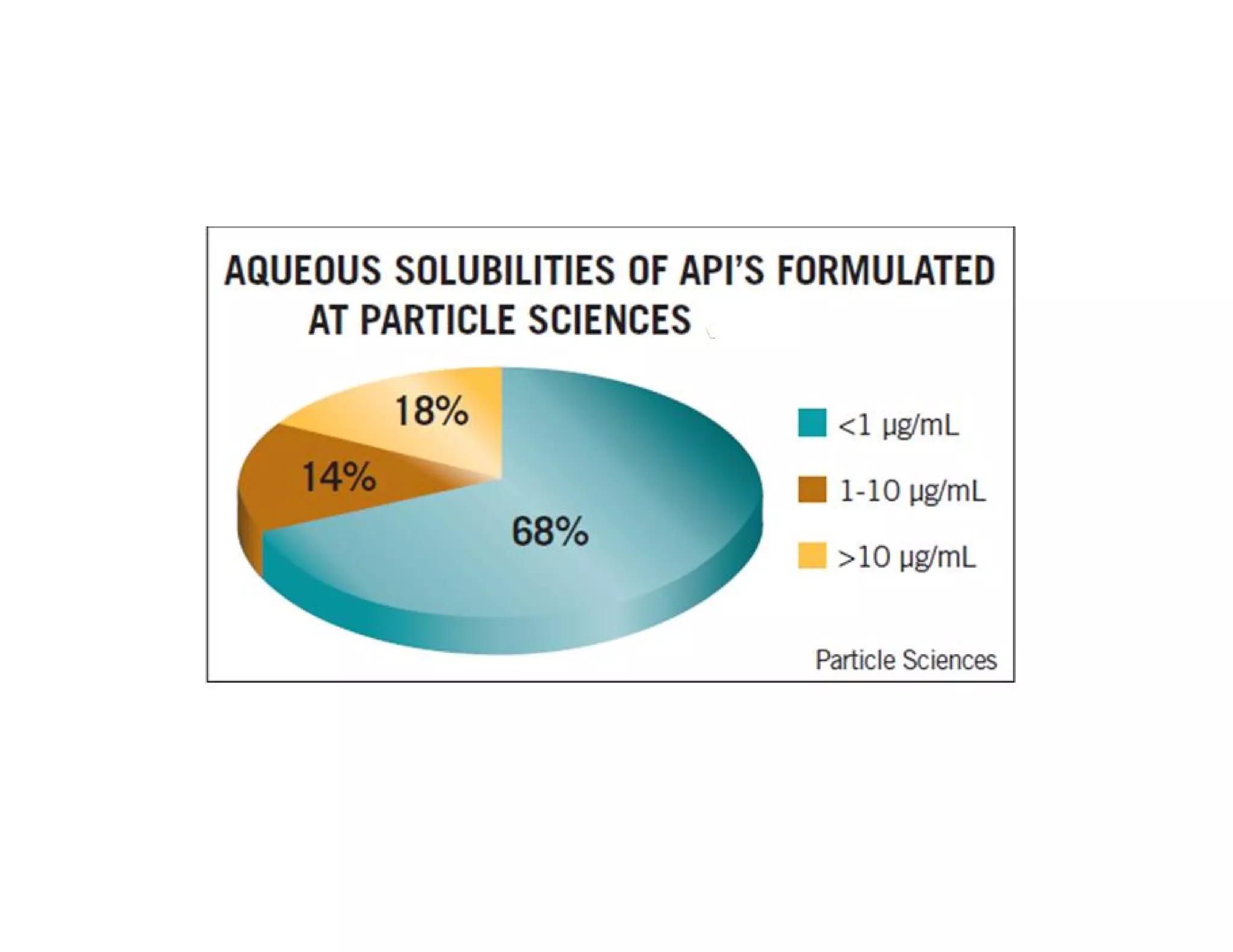
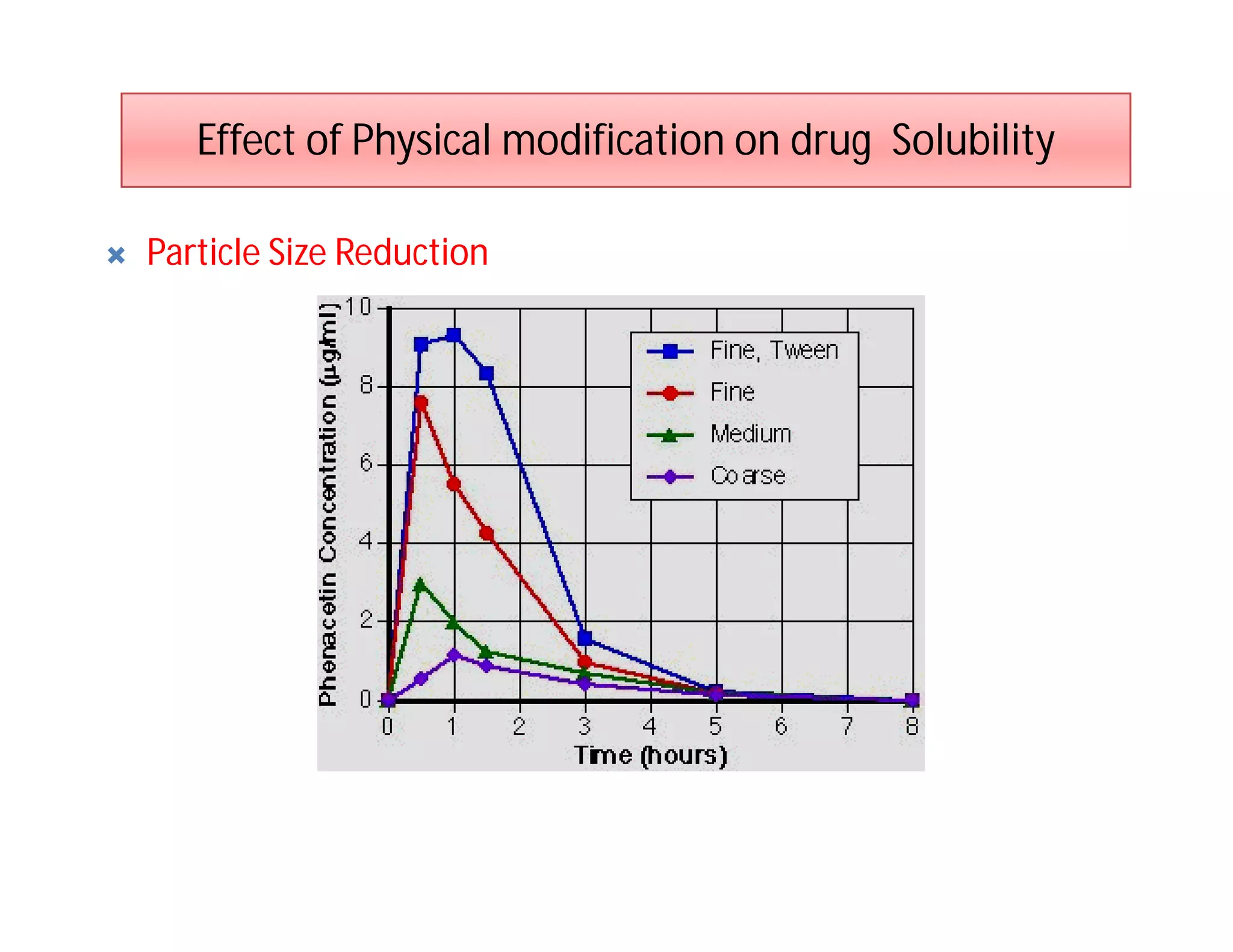
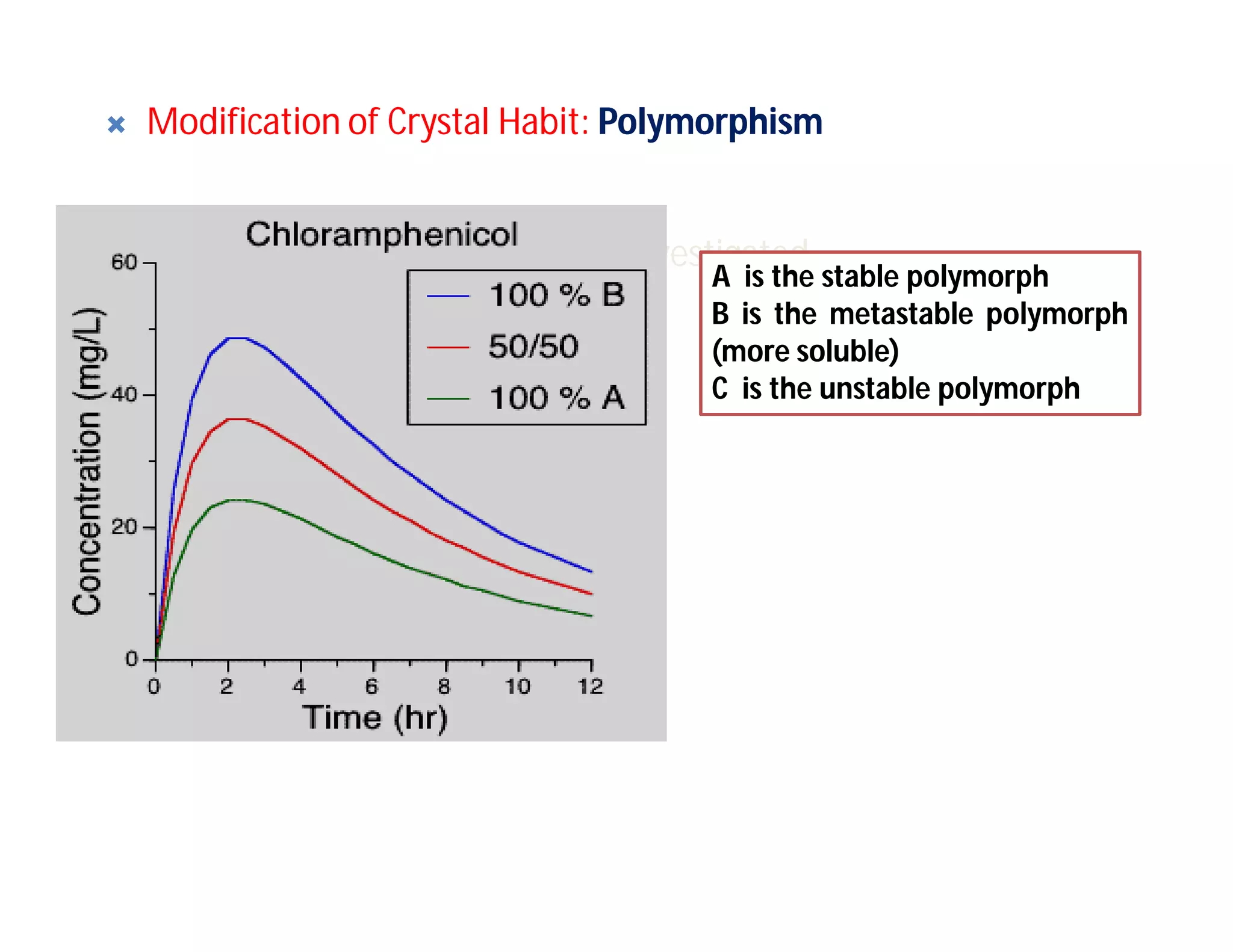
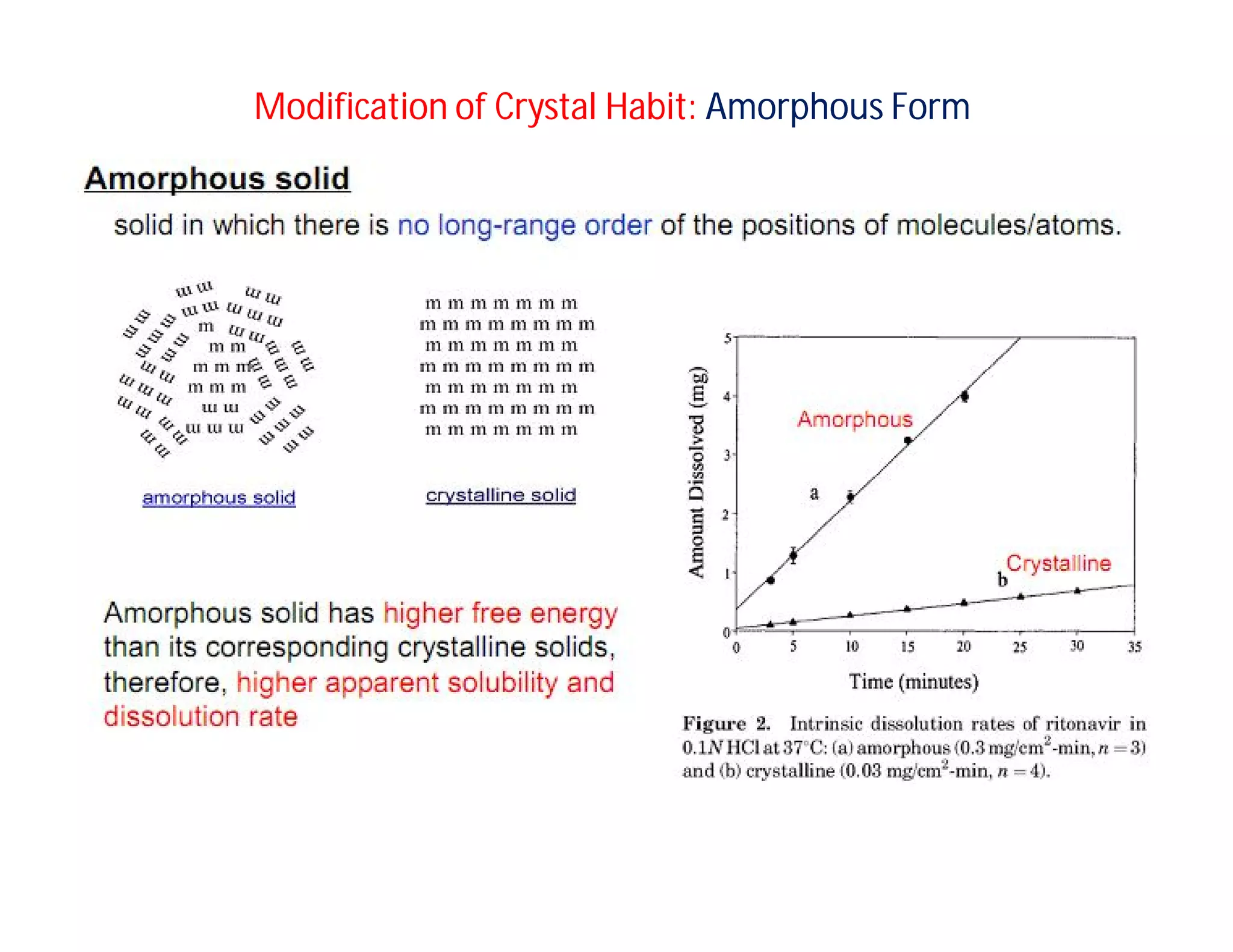
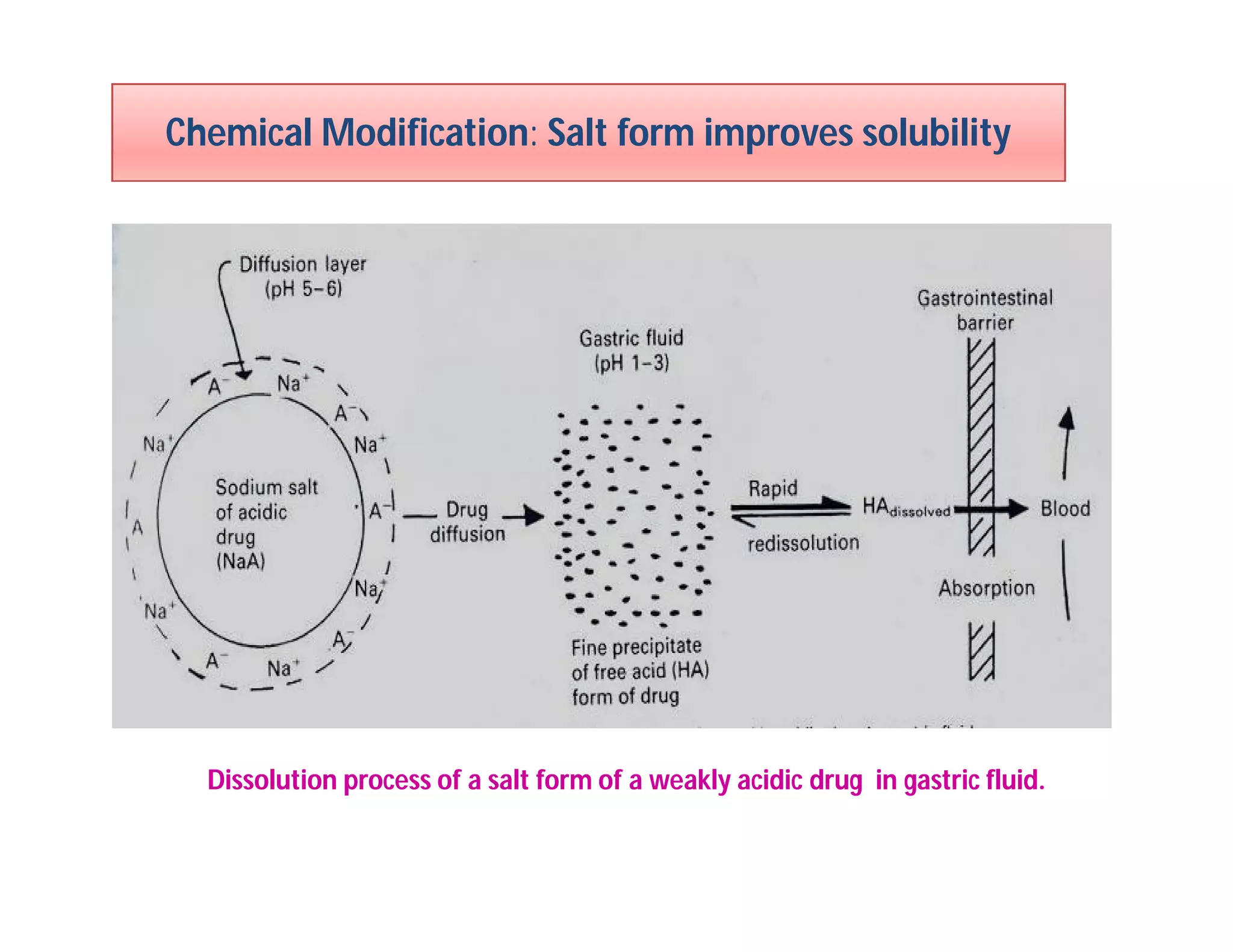
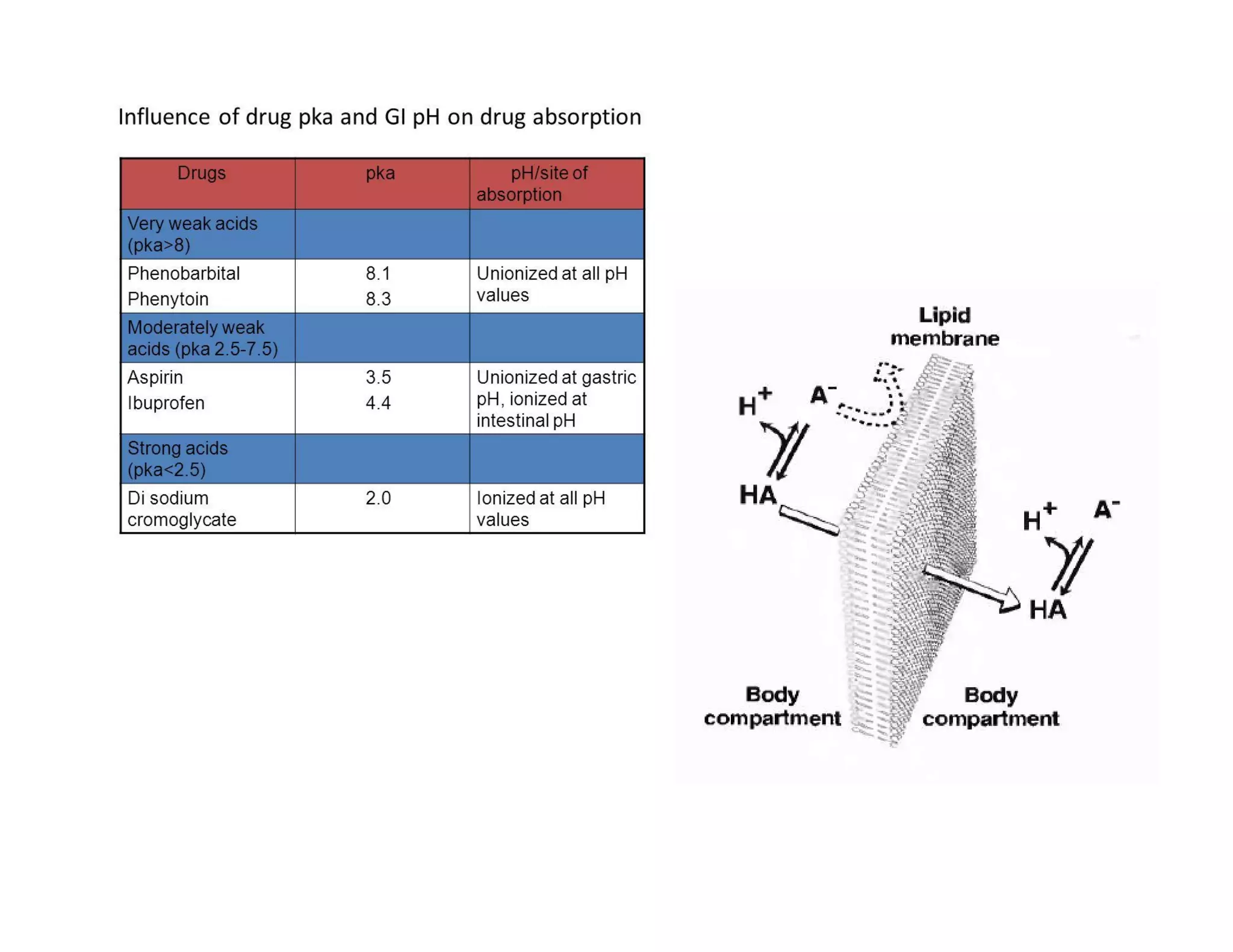
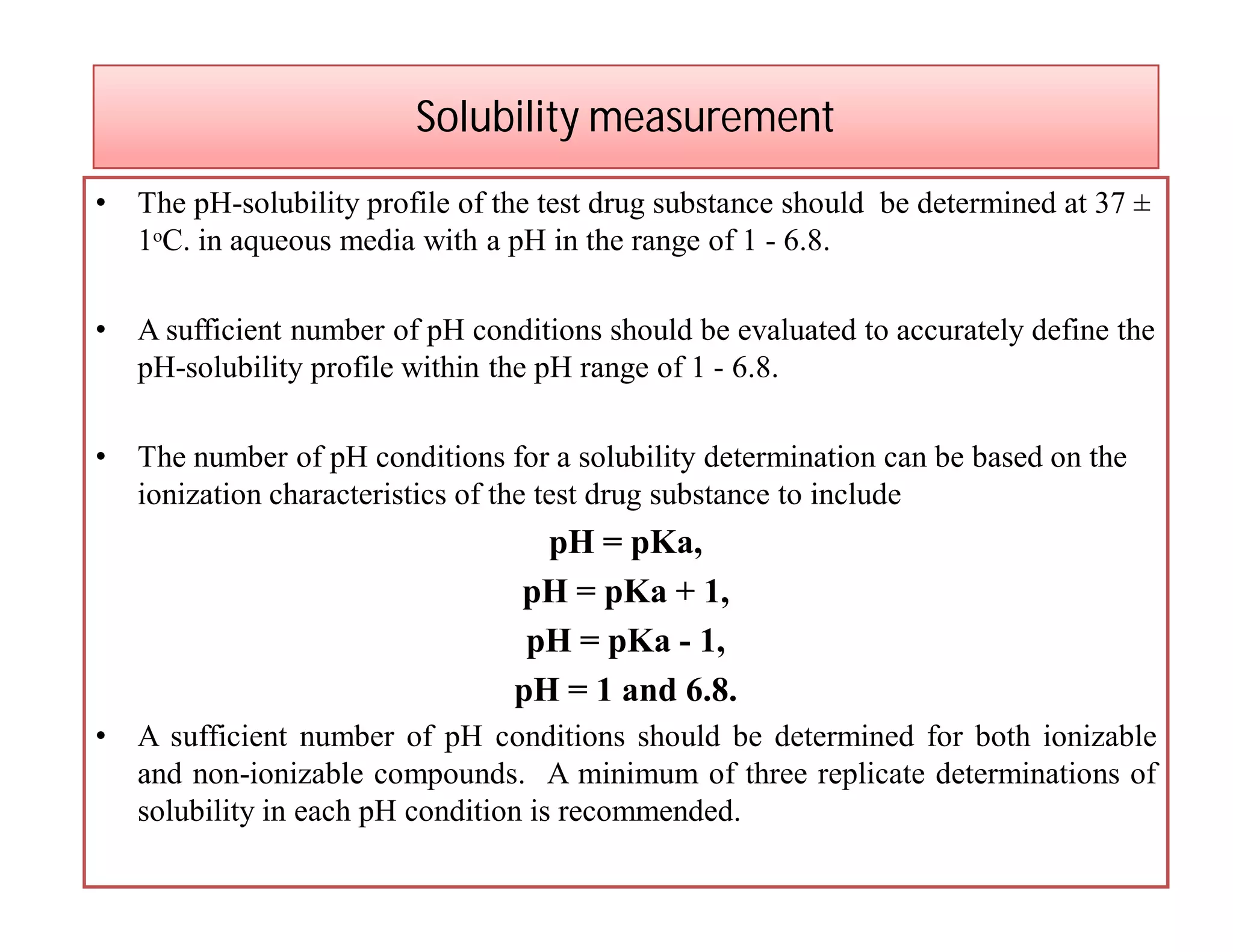
Dr. Nilesh Kulkarni discusses solubility and the Biopharmaceutics Classification System (BCS). The BCS classifies drugs based on their aqueous solubility and intestinal permeability. It divides drugs into four classes. Solubility can be improved through methods like reducing particle size, modifying crystal habit through polymorphism or amorphous forms, or forming salts. A drug's solubility is important for its dissolution and absorption.