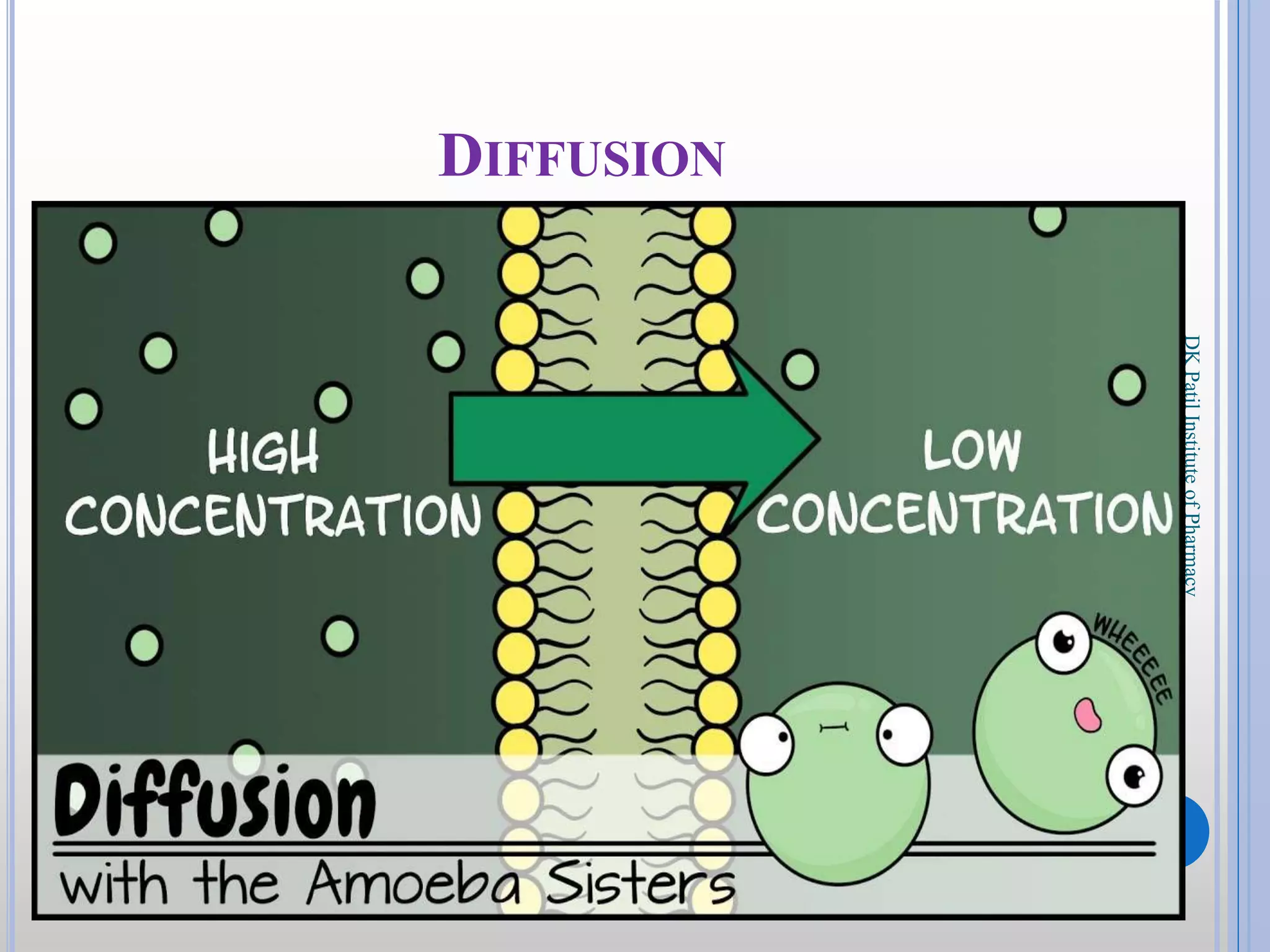
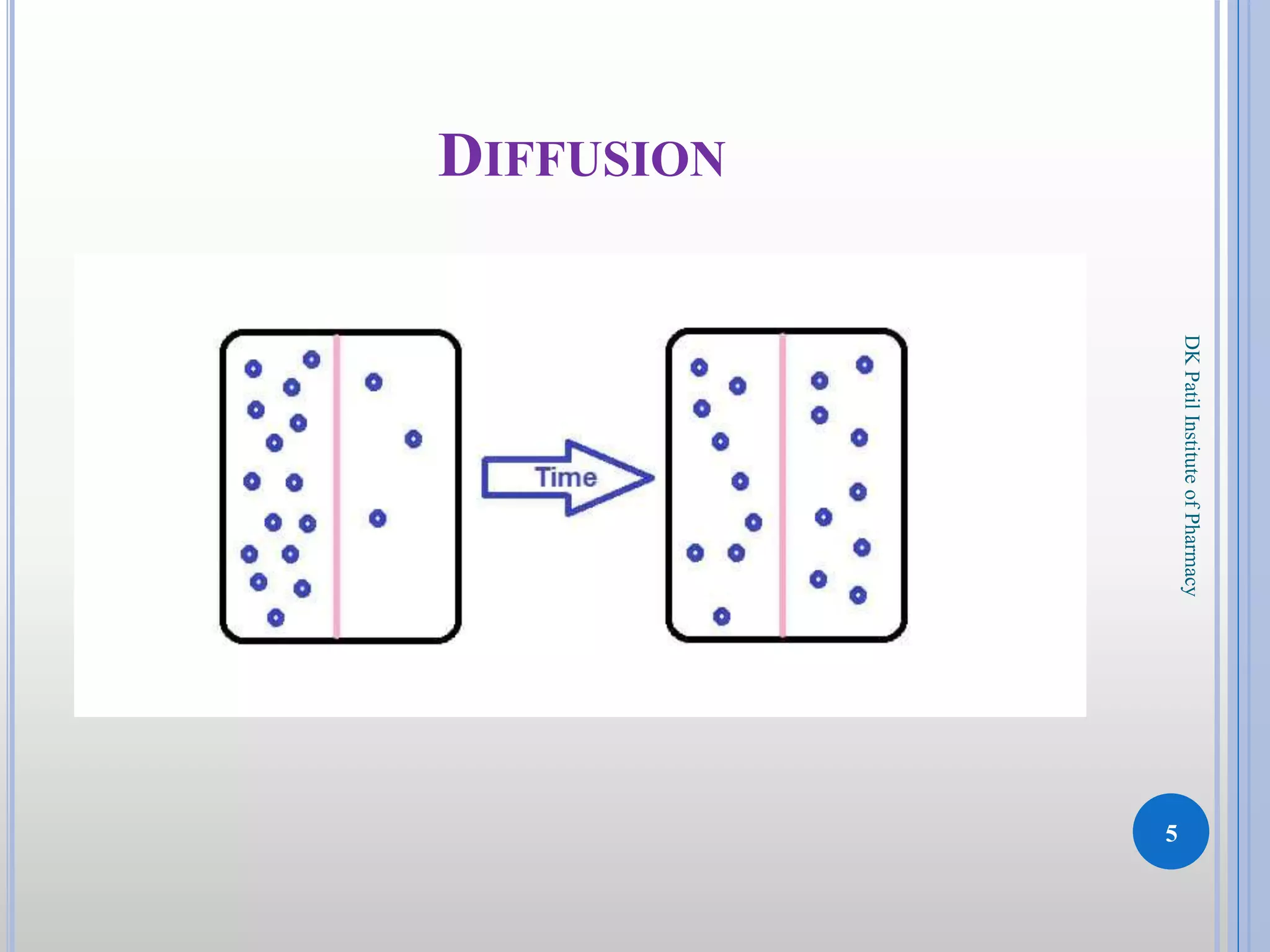
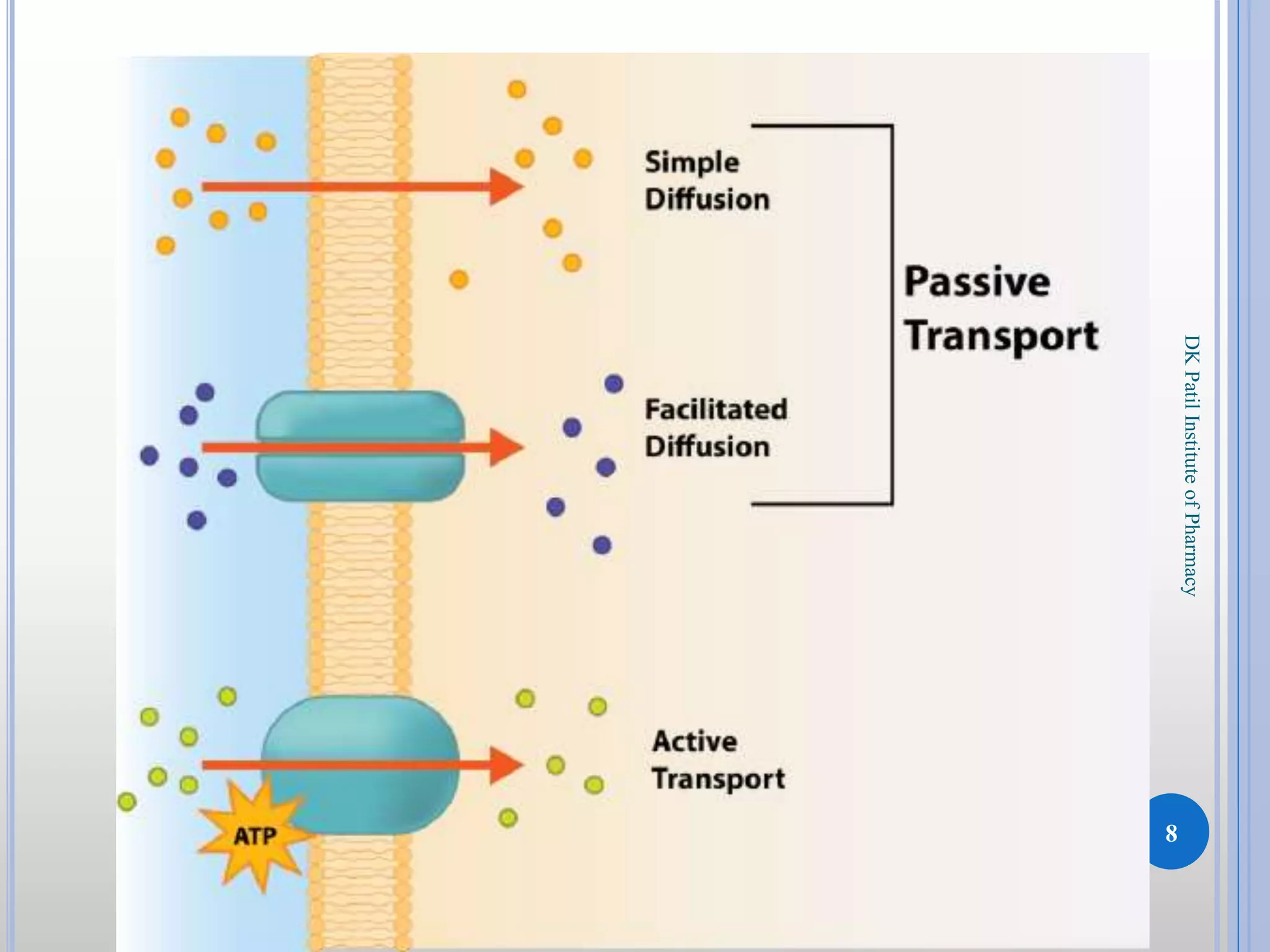
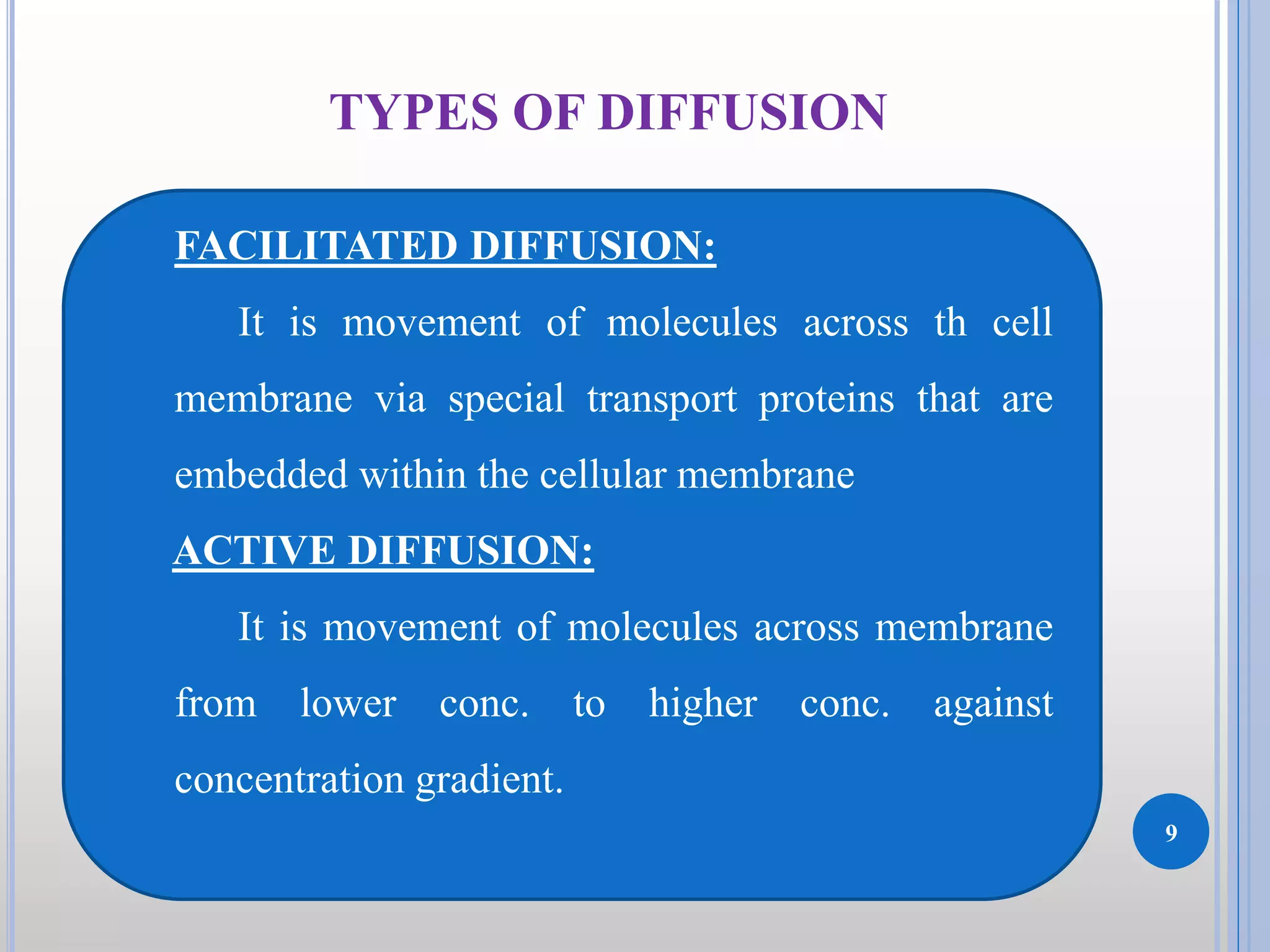
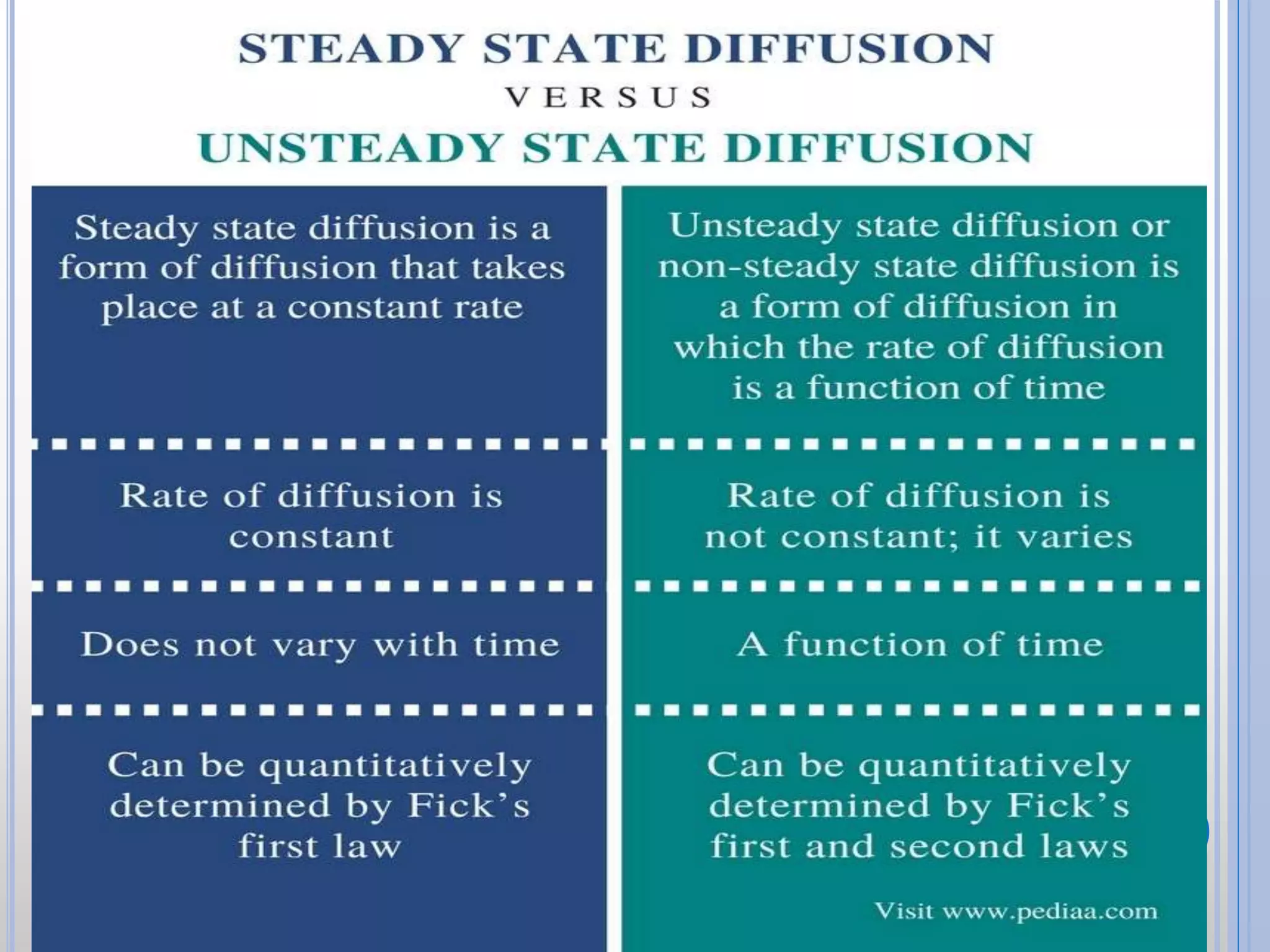
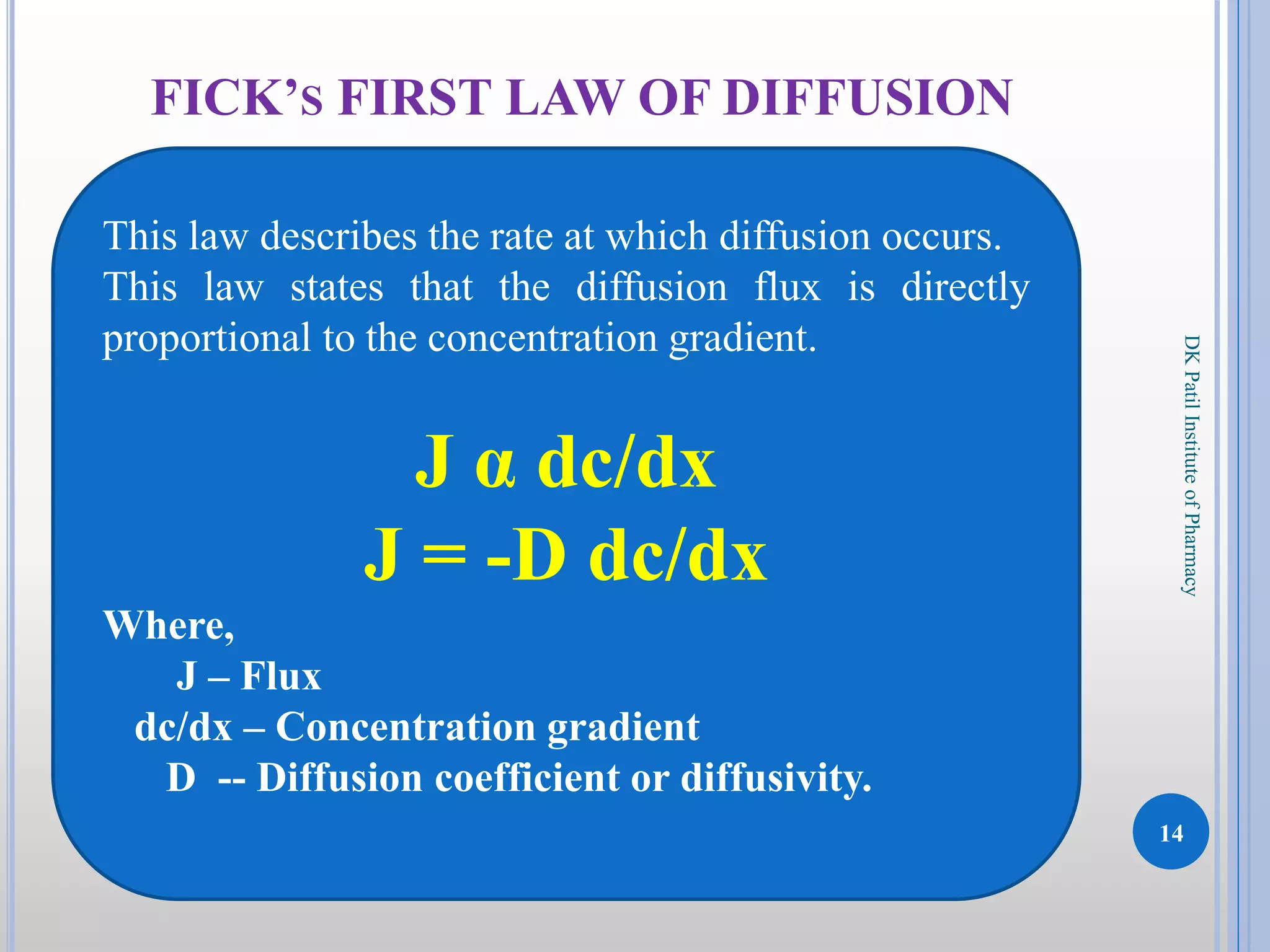
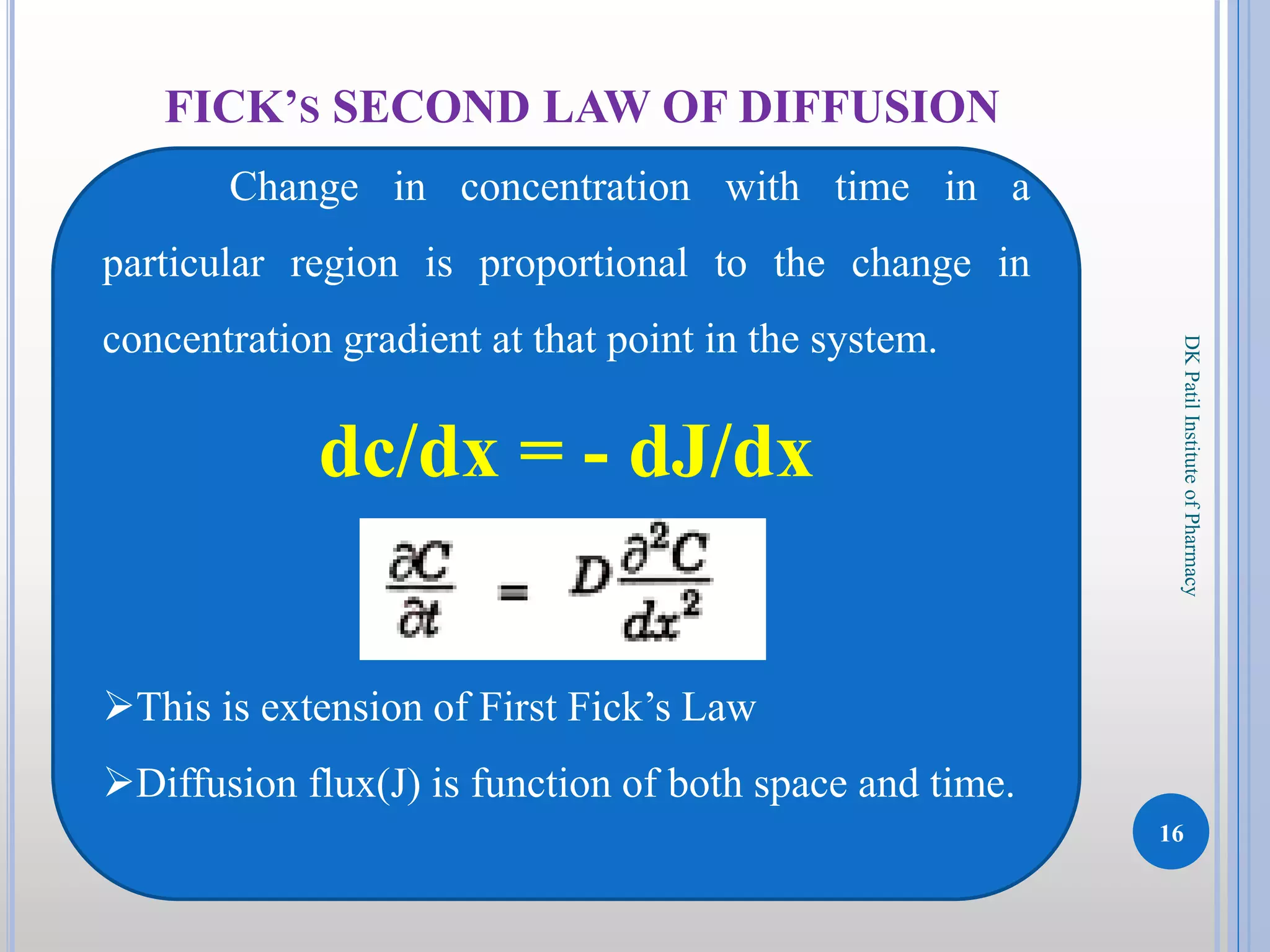
The document discusses the principles of diffusion in biological systems, explaining its definition, types, and significance, particularly in drug release and distribution. It covers Fick's laws of diffusion, which detail the relationship between diffusion flux and concentration gradients, noting key differences between passive, facilitated, and active diffusion. Overall, the content highlights how diffusion processes are critical to various biological and pharmaceutical applications.