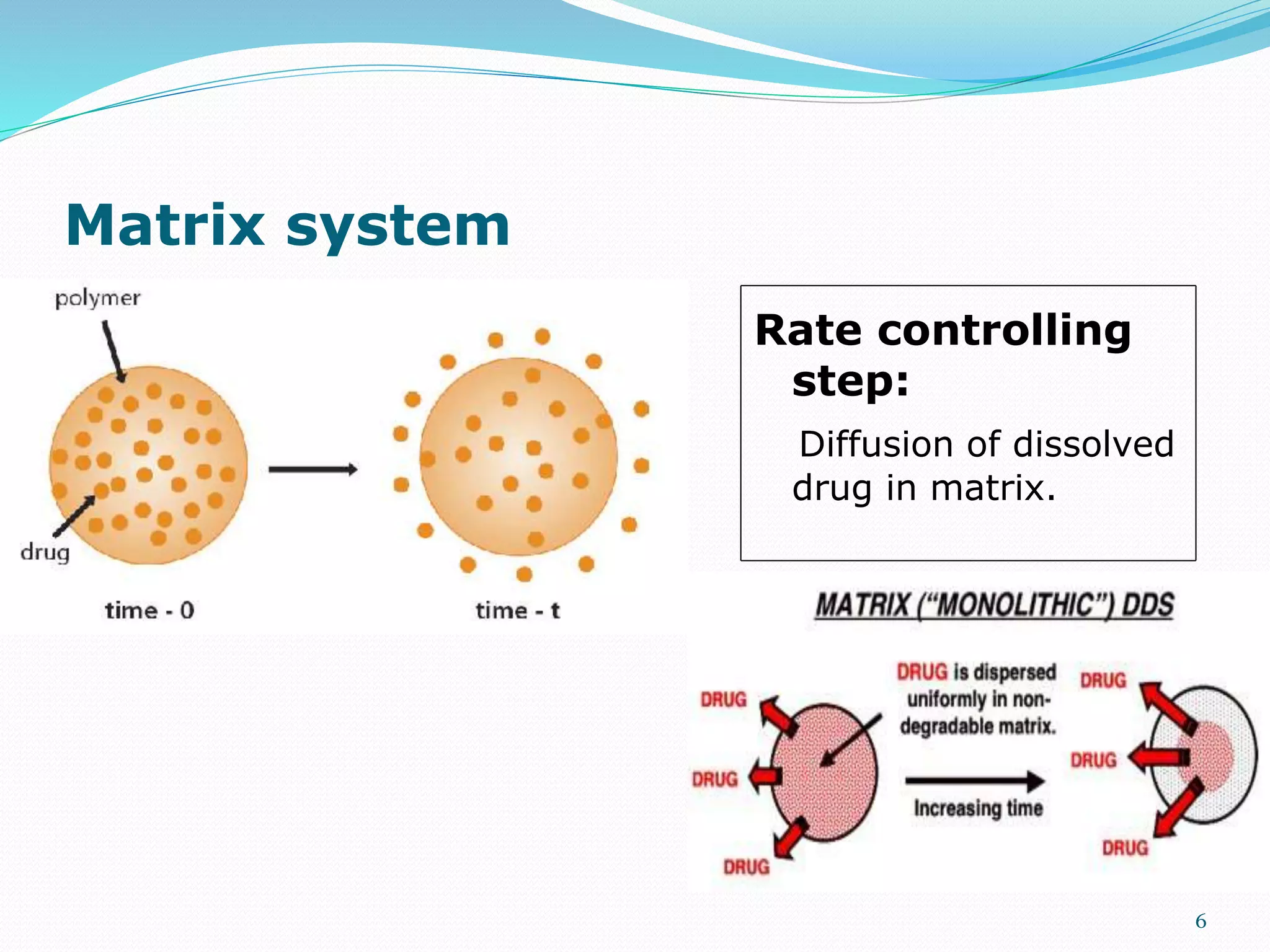
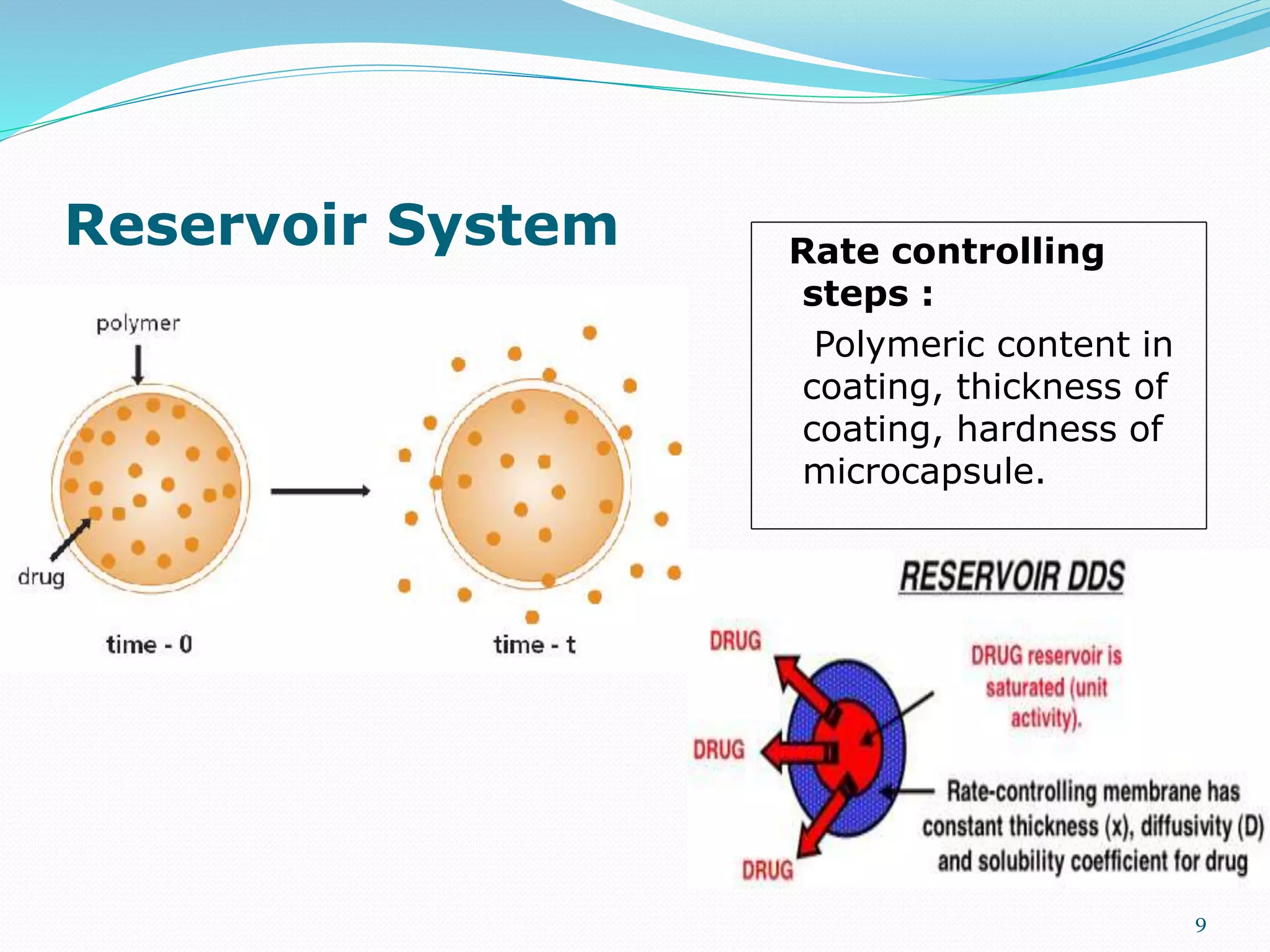
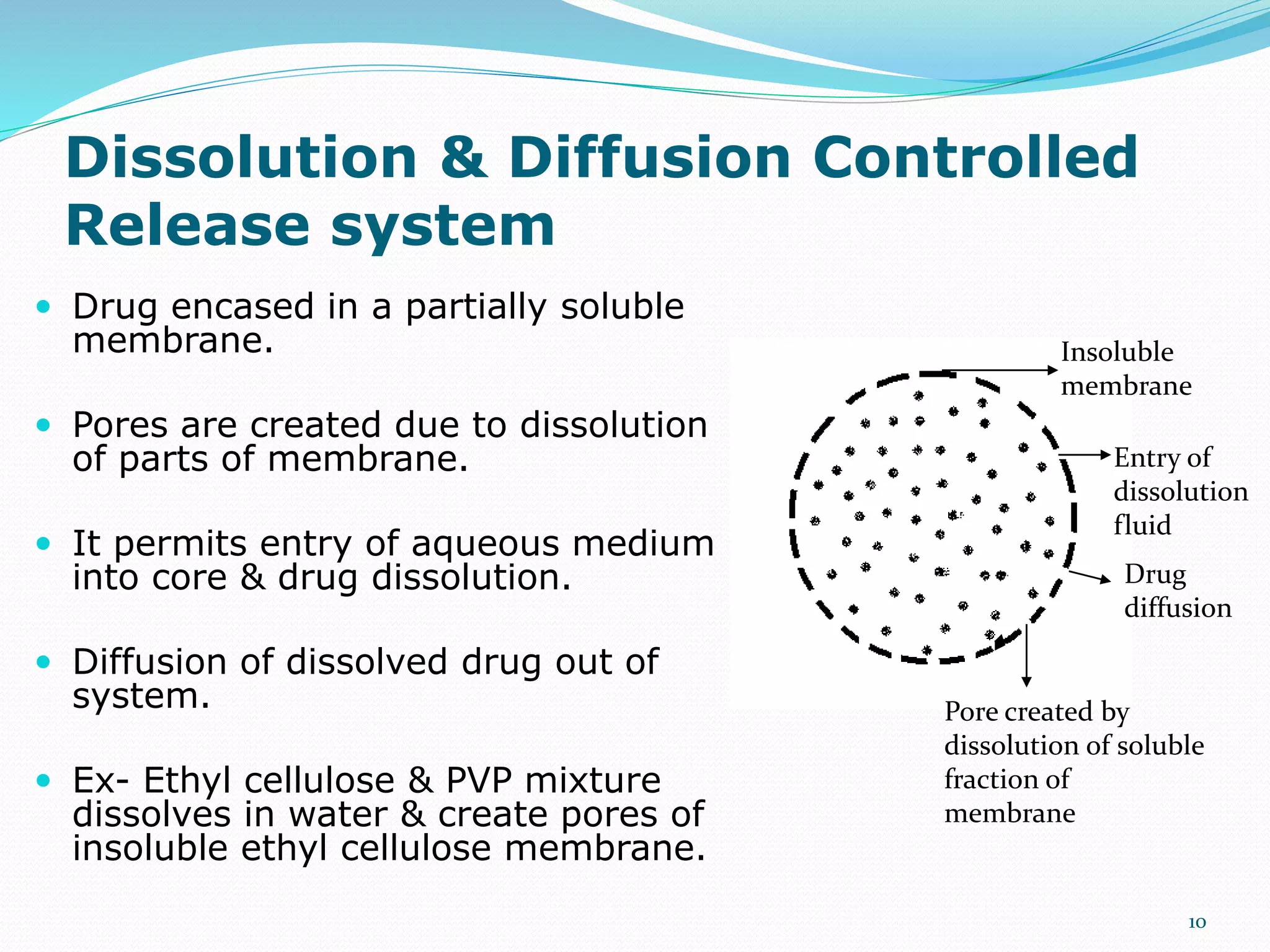
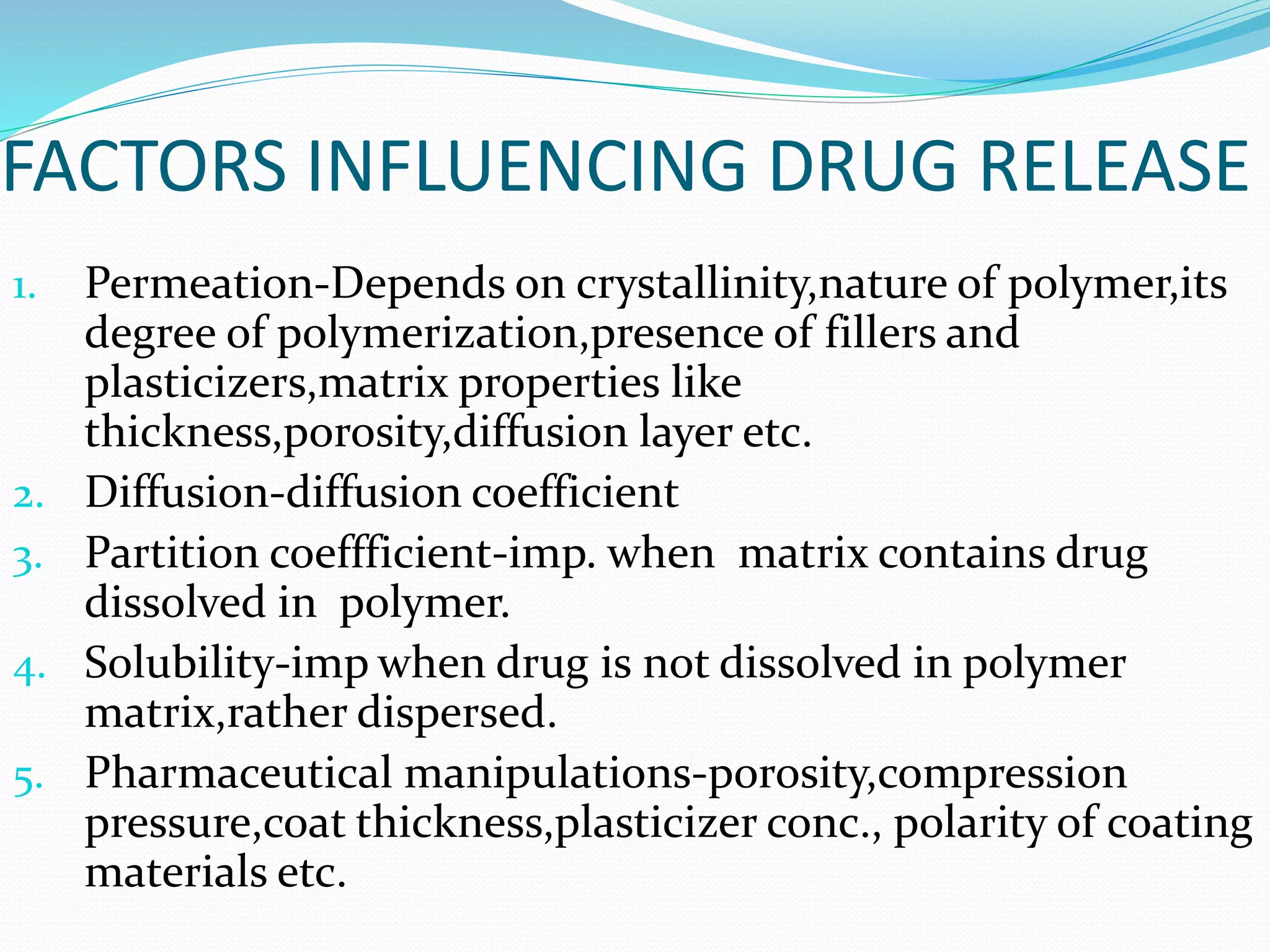
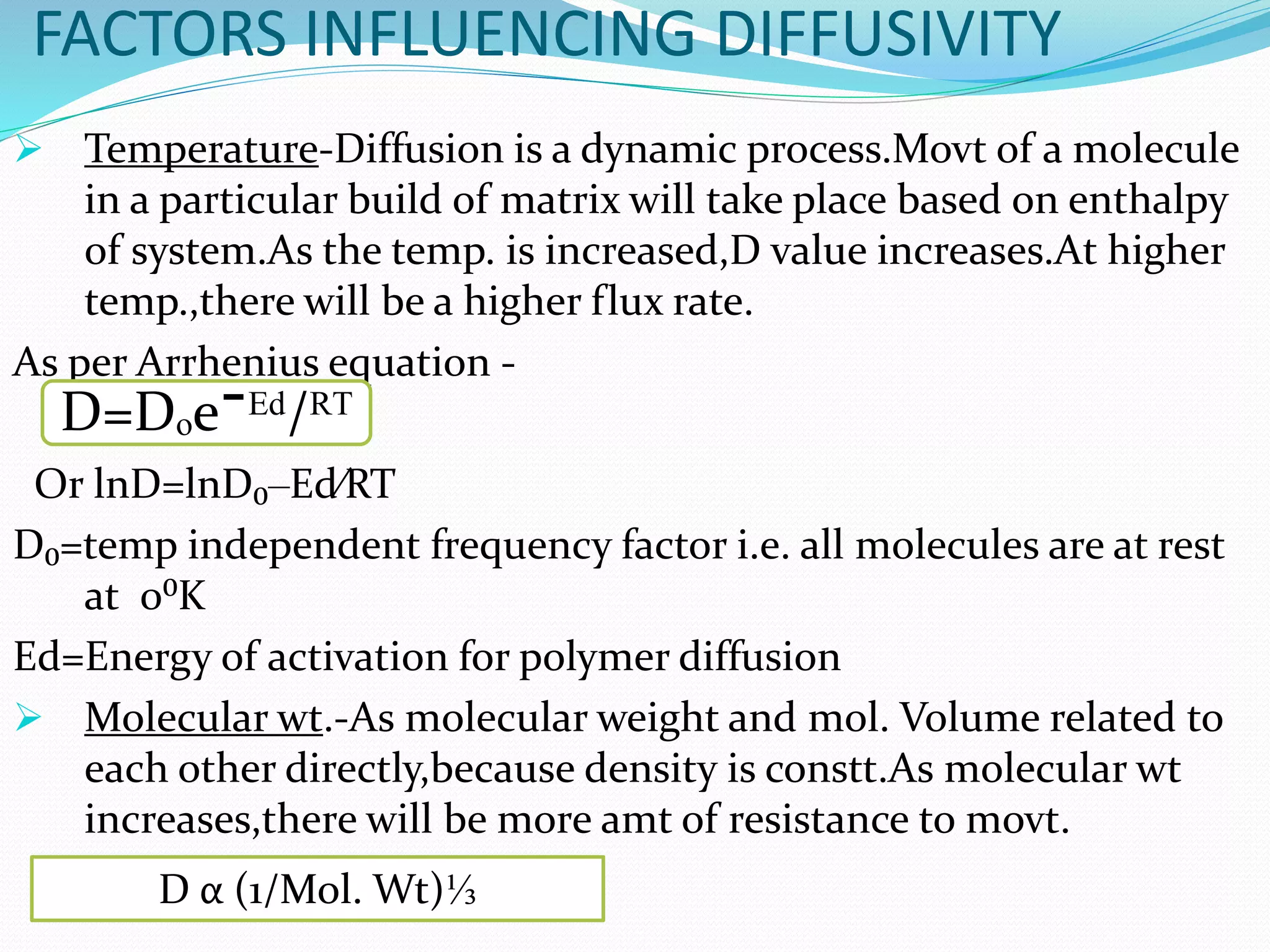
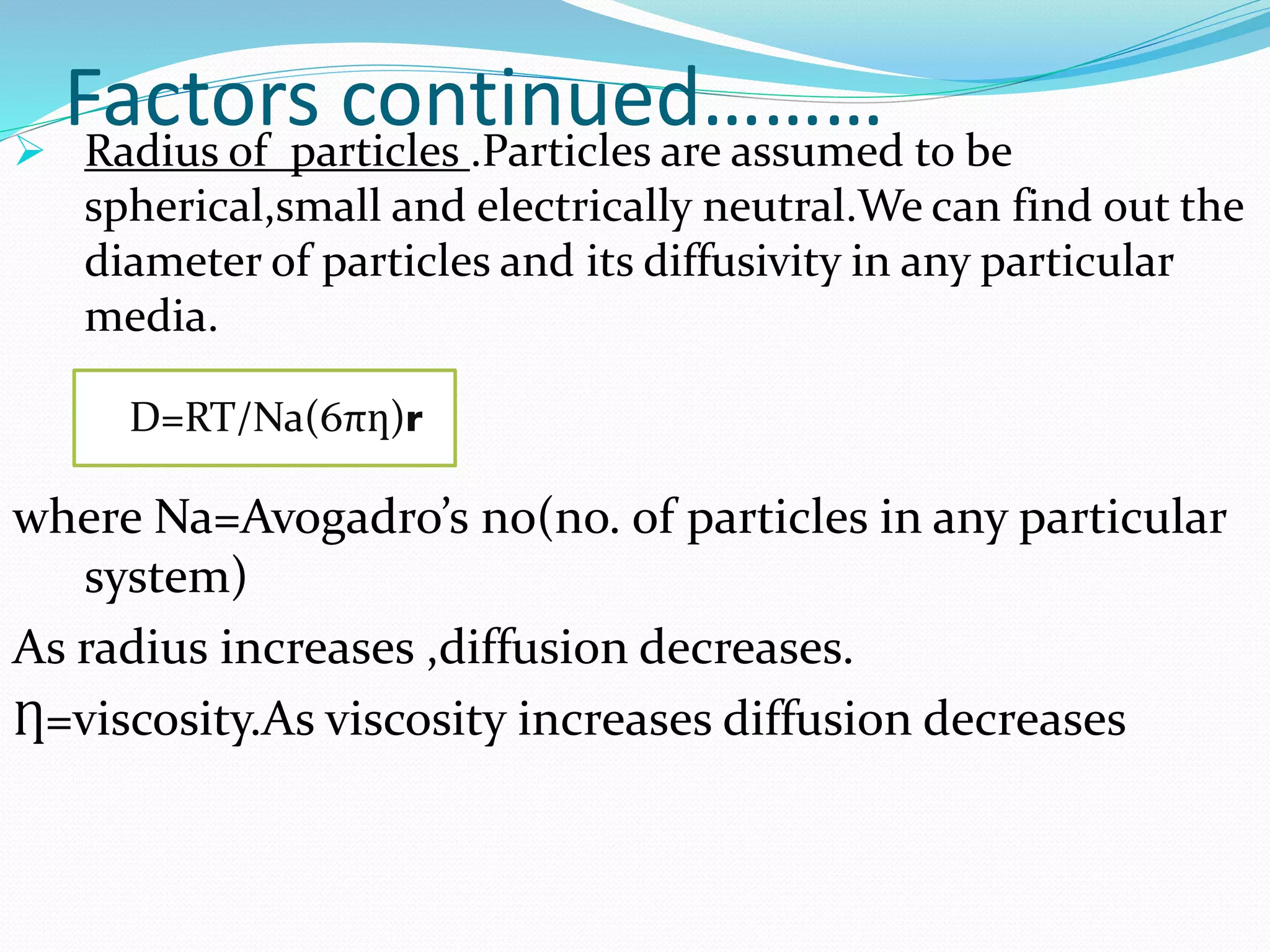
This presentation discusses kinetics of drug release from controlled release drug delivery systems. It describes different types of controlled release systems including matrix systems, reservoir systems, and dissolution-diffusion controlled systems. Rate controlling steps and drug release mechanisms such as diffusion, erosion, and dissolution are discussed. Factors influencing drug release and diffusion such as temperature, molecular weight, particle size, and viscosity are also summarized. Finally, mathematical models for drug release kinetics including zero-order, first-order, Higuchi, and Korsmeyer-Peppas models are presented.







![In first case amt. in core changes but conc remains same inside
upto a particular time and undergoes dilution so that dC
becomes constt. So when concentrations are changing with
time,as in the case of above experiment,we may know dC/dX
at the beginning of experiment,but the mass flow will
continually be changing the conc. gradient.Therefore it is
necessary to introduce time as a variable.Eqn then becomes-
This equation is called Fick’s 2nd law of diffusion.
Interpretation from the equation-
Rate of change in conc. in volume element is proportional to
area of change of conc. gradient in that region of field.
Diffusion coefficient(D) is a measure of rate of drug movt.
J=[dC/dt]x=D[d²C/dX²]ᵼ](https://image.slidesharecdn.com/70ac74f8-9126-4c94-812c-05fc071a2e97-160223112820/75/applicationsofdrugreleasedata-130212133522-phpapp01-14-2048.jpg)

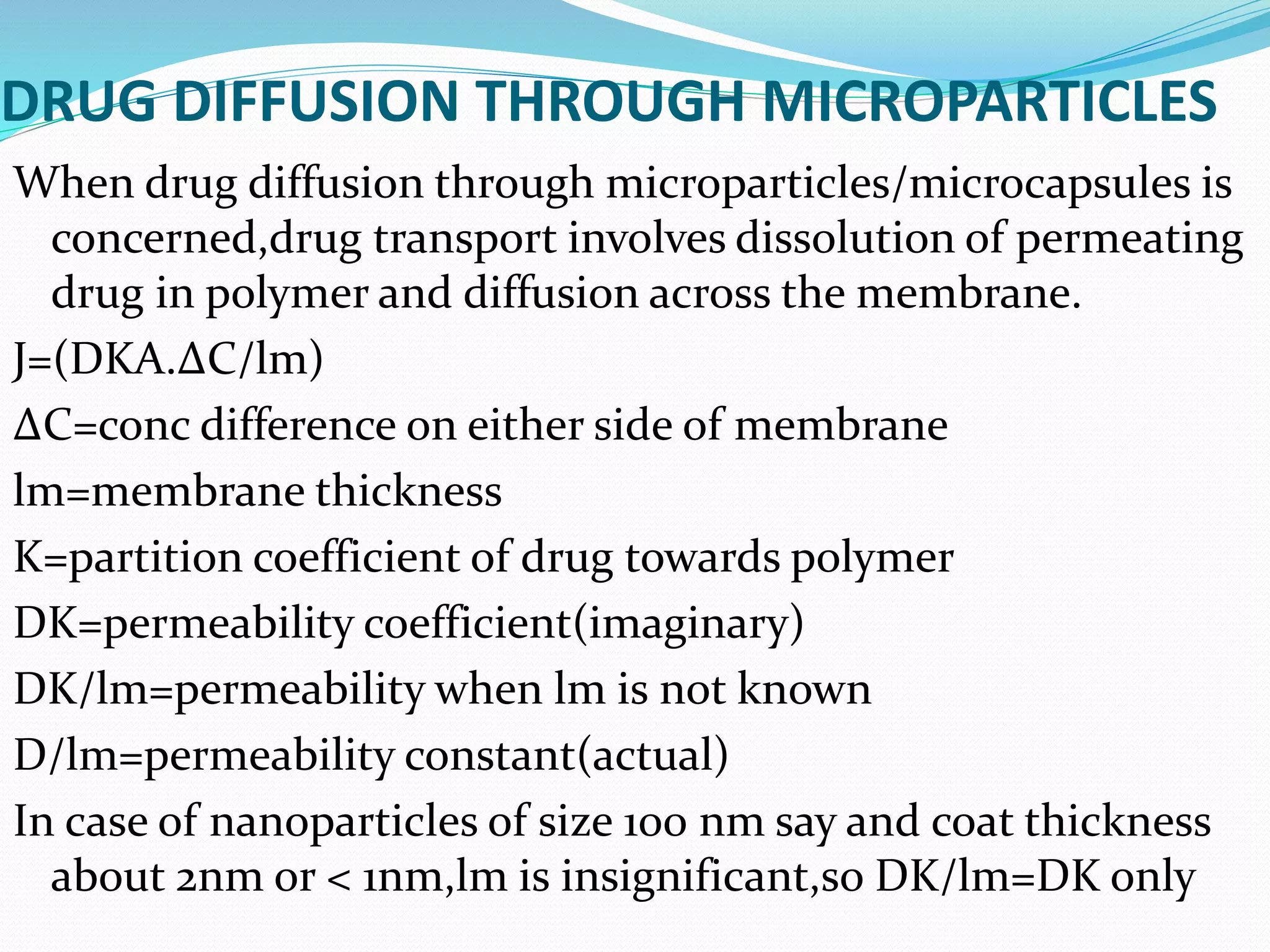
![Si-Nang and Carler eqn for drug release
from microcapsules
dC/dt= [DsAK/Vlm]
Where A= internal surface area of coating.
K= Porosity and tortuosity.
Mechanisms/ Mathematical models of
drug release
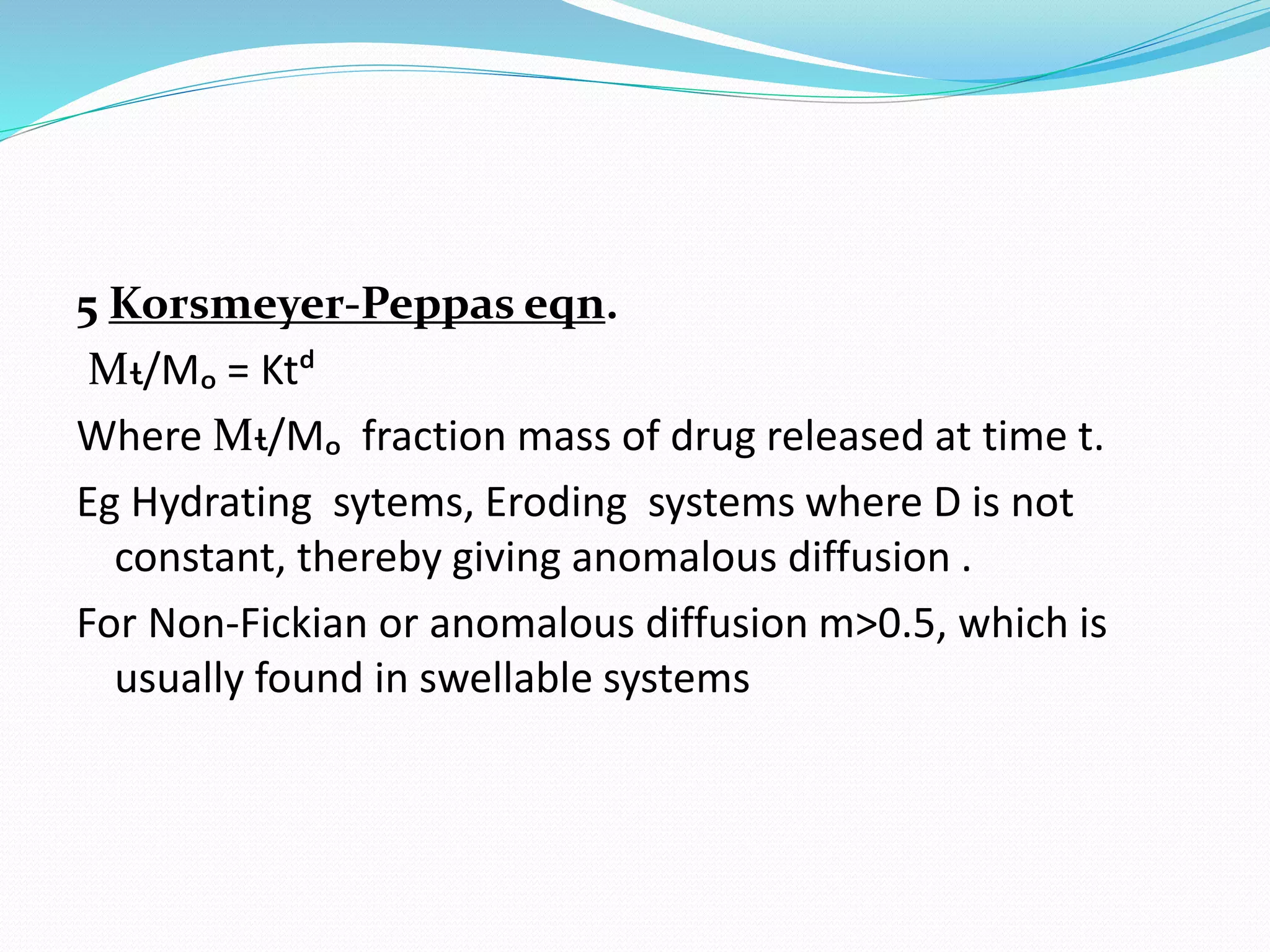
1. First order
ln Xt = ln Xo+Kt (Release proportional to amount of
drug remaining )
Systems that follow the model – Water soluble drugs in
porus metrix](https://image.slidesharecdn.com/70ac74f8-9126-4c94-812c-05fc071a2e97-160223112820/75/applicationsofdrugreleasedata-130212133522-phpapp01-19-2048.jpg)