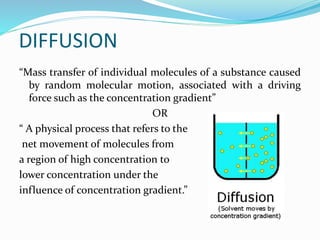
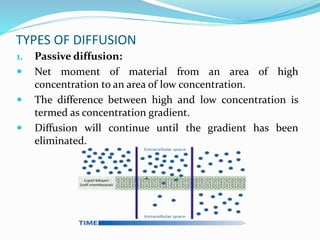
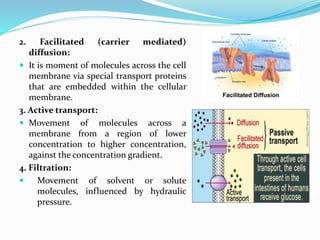
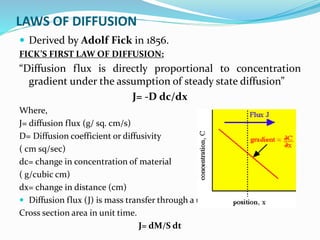
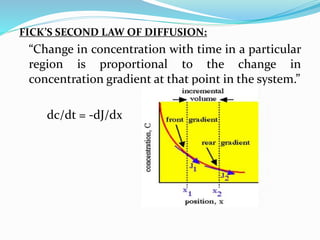
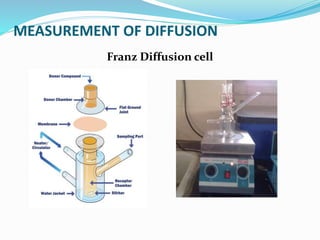
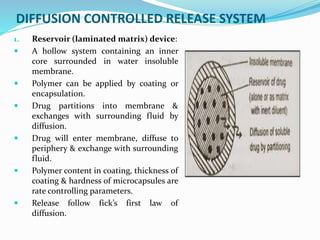
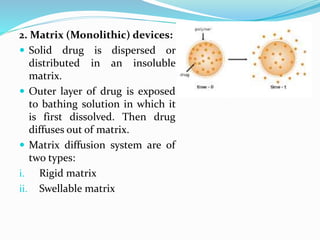
This document discusses diffusion principles in biological systems. It defines diffusion as the mass transfer of molecules caused by random molecular motion down a concentration gradient. There are different types of diffusion, including passive diffusion, facilitated diffusion, active transport, and filtration. Fick's laws of diffusion state that the diffusion flux is directly proportional to the concentration gradient, and the change in concentration over time is proportional to the change in the concentration gradient. Diffusion can be measured using a Franz diffusion cell, which contains a donor chamber with a known solute concentration separated from a receptor chamber by a membrane. The amount of solute diffusing through the membrane over time is then measured. Diffusion-controlled release systems include reservoir devices with a drug-loaded core