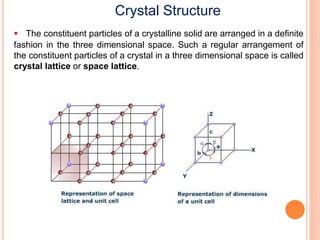
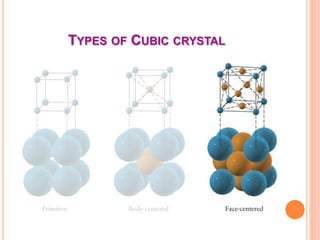
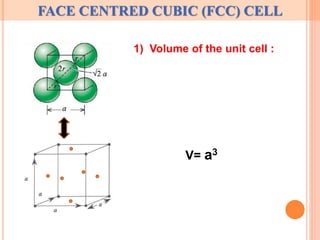
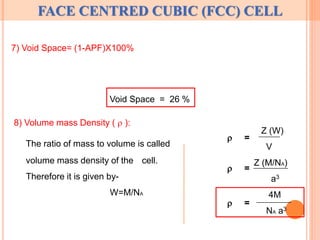
The document discusses properties of a face centered cubic (FCC) crystal structure. An FCC crystal has:
1) A unit cell with 4 atoms located at the corners and face centers of the cube.
2) An atomic radius of a/√22 where a is the lattice parameter.
3) A nearest neighbor distance of a/2 between adjacent atoms.
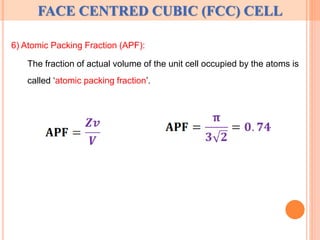
4) A coordination number of 12, atomic packing fraction of 0.74, and void space of 26%.